Is Nitrogen polar or nonpolar
Nitrogen (N2) is essential for all living beings on this planet. Nearly ninety-eight percent of the world’s Nitrogen is found in the solid earth within the chemical structure of rocks, sediments, and soils. Around eight metric tons of nitrogen cover every unit square meter of the earth. Nitrogen in the molecule form is stable and helps to convert Nitrogen to other chemical compounds with the intake and giving out of a specific amount of energy. This compound was discovered by Daniel Rutherford in 1772. It is a colorless and odorless gas that comprises 78% of our atmosphere.
Nitrogen is considered the 5th most commonly found element in the world. The atomic number of this compound is 7, and its atomic mass is 14.0067. The Melting Point of Nitrogen is -345 degrees Fahrenheit, roughly equal to -210 degrees Celsius. The Boiling Point of Nitrogen is -320.5 degrees Fahrenheit, approximately equal to -196 degrees Celsius. The density of Nitrogen is 0.0012505 grams per cubic. Nitrogen exists in the form of gas at room temperature and belongs to the class or group of Nonmetals.
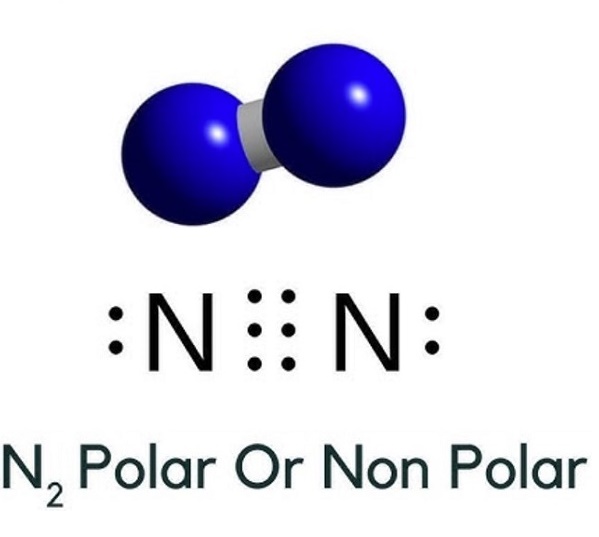
Geometry and shape of N2 molecule: In N2, both Nitrogen atoms form a bond to complete the octets. The molecule has linear geometry as it is arranged in the same plane.
The difference in electronegativities of atoms in N2: N2 comprises 2 Nitrogen atoms. The electronegativity value for the Nitrogen atom is 3.04, which will be the same for both atoms forming a bond in the molecule. And as this molecule has no difference in electronegativities, there will be no uneven distribution of charges. There will be no partially charged regions formed in the molecule.
Net dipole moment in N2: Both atoms have equal electronegativity and share a similar proportion of charge, and the overall molecule results in a net-zero dipole moment.
In summary, the Nitrogen molecule is a non-polar covalent molecule. It has zero dipole moment. Two N atoms in the nitrogen molecule have zero electronegativity difference. The bond pairs of electrons are equally distributed between two N atoms.