What is the ionic charge when iodine forms an ion?
Iodine, symbolized as 'I,' is a dark grey or purple-black nonmetallic element with atomic number 53 on the Periodic Table. Despite its ability to form compounds with many elements, it is the least reactive and most electropositive halogen.
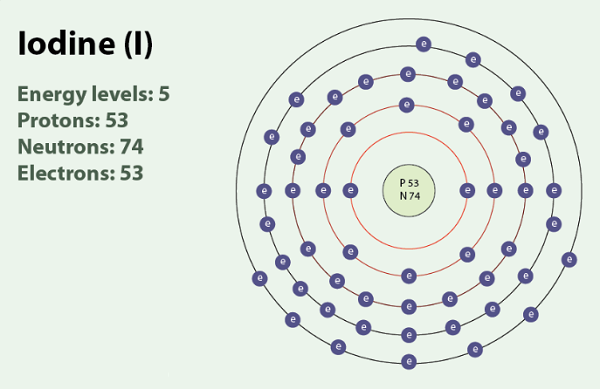
In its neutral state, the iodine atom typically possesses seven valence electrons. However, gaining one electron achieves a stable octet configuration, similar to noble gases. This electron acquisition results in the formation of the iodine ion, denoted as I-, which carries a charge of -1. This ion plays a crucial role in numerous chemical processes and reactions. The negative charge of the iodine ion signifies an extra electron compared to its neutral state, contributing to its distinct characteristics and behavior. Iodide, an anion with a valency of -1, refers to iodine compounds with an oxidation state of -1.
Iodine is primarily found on Earth as water-soluble iodide in brine pools and oceans. When heated, it emits a purple color and exhibits limited solubility in water, dissolving only in select solvents like carbon tetrachloride. Naturally occurring iodine is present in the air, soil, and water, with oceans serving as its most significant source. Certain minerals also contain iodine, essential for proper brain function in humans.
Additionally, iodine exists as polyatomic ions, including the Hypoiodite Ion, Iodite Ion, Iodate Ion, and Periodate Ion.
You may like
Related articles And Qustion
See also
Lastest Price from iodine manufacturers

US $360.00-99.00/kg2025-04-21
- CAS:
- 12190-71-5
- Min. Order:
- 1kg
- Purity:
- 99% purity,WhatsApp+86 18102676775
- Supply Ability:
- 20 tons

US $23.00-1.00/kg2025-03-07
- CAS:
- 12190-71-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons