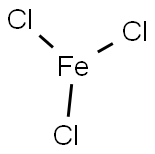
What is the charge of the metal ion in FeCl3?
Ferric chloride is named as iron chloride. It is a chemical compound with a chemical formula consisting of FeCl3. The charge on the iron cation in iron(III) chloride has a charge of 3+.
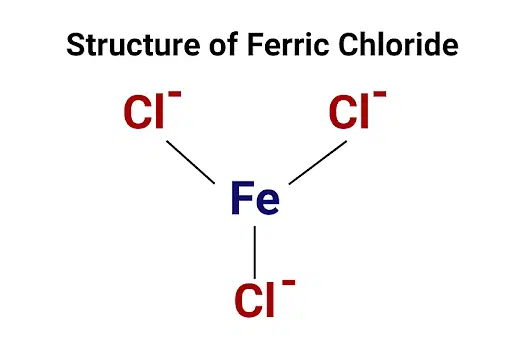
Fe Iron has oxidation numbers of +2 and +3. Combining with Iron Chlorine will have a negative charge Oxidizing the Iron. The most likely charge of the Iron is +3 which gives the Chlorine a charge of -1. Ferric chloride is a common compound of iron and chlorine in which iron possesses +3 oxidation state.
It has a relatively low melting point as compared to its boiling point, which is around 315 degrees Celsius. When dissolved in water, ferric chloride goes through the hydrolysis process and gives off heat in an otherwise exothermic reaction. It is produced in industries by the reaction of dry chlorine with scrap iron at 500 to about 700 degrees Celsius temperature .
You may like
Related articles And Qustion
Lastest Price from Ferric chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7705-08-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 TON

US $0.00-0.00/kg2025-03-07
- CAS:
- 7705-08-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons