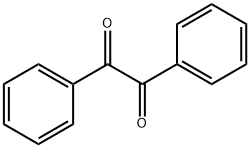
Nitration and reactivity of benzoin
Introduction
The benzil-cyanide reaction is a cyanide-specific reaction that has been exploited to produce a colorimetric indicator for this toxic anion. This was done by producing a π-extended analogue of benzil, which is soluble in a 70:30 (v/v) mixture of methanol-water. In this medium, dilute solutions of benzil are yellow but produce colorless products when exposed to low concentrations of cyanide anion (g1.7 µM; added as an aqueous NaCN solution), but no other common anions (e.g., OH-, F-, N3 -, benzoate-, and H2PO4 -). On the basis of these observations and supporting mechanistic analyses, it is concluded that the modified benzil system benzil is a promising cyanide anion indicator that is attractive in terms of its selectivity, ease-of-use, water compatibility, and the low, naked-eye discernible cyanide detection limit it provides[1].

Picture 1 Benzoyl powders
Structure
The unit cell and space group of benzil, C6H5. CO. CO. C6H5, were first determined by Becker & Rose (1923) who obtained a=8.15 and c--13.46 A in the trigonal space group D~ and D~. These values were improved somewhat by Allen (1927). Later, benzil crystals were found to give a marked diffuse scattering pattern (Lonsdale & Smith, 1941) which became very much reduced at low temperatures. The most promi[1]nent feature of this diffuse pattern was a set of streaks along layer lines. In order to interpret this effect fully, it was necessary to know the structure of the crystals, and for this reason the present work was undertaken.
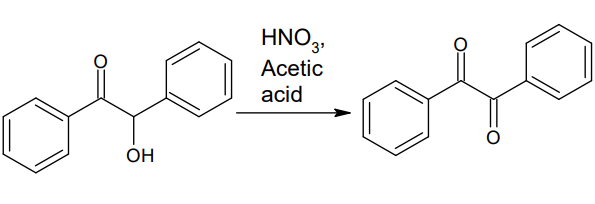
The nitration of benzoin
Since the first observation over one hundred years ago, that benzoin could be oxidized to benzil by the use of chlorine’ or nitric acid, many other procedures have been developed. Of these, the use of copper sulfate and pyridine as a modified Fehling solution, which may be regenerated by air, is of recent interest. Earlier a catalytic method had been reported using copper oxide at high temperature. In 1951, Bacharach and Brolles, Jr.,6 attempted the nitration of benzoin using lithium nitrate in acetic anhydride and un[1]expectedly obtain benzil in poor yields. These yields were improved, somewhat, by the addition of cupric nitrate. Independently, in 1941, during a study of the action of ammonium salts in glacial acetic acid, it was found that a smooth conversion of benzoin to benzil could be achieved by the use of a large excess of ammonium nitrate, which, however, did not react completely when over 0.5 mole of benzoin was used.? A satisfactory preparative method has now been found to oxidize benzoin and other acyloins to the corresponding diketones in 90% to quantitative yields, by the use of a catalytic amount of a cupric salt, which may be regenerated internally by am[1]monium nitrate. Since this reaction is run in acetic acid, the solvent performs a dual role by decomposing the formed ammonium nitrite, while serving as an excellent medium to crystallize the diketones. The reaction may be fairly specific for a-hydroxy ketones, since neither benzasde[1]hyde, nor benzohydrol nor mandelic acid is oxidized under these conditions. Iron, cobalt and nickel salts have been found to act equally as catalysts[2].
The reactivity of benzil
The reactivity of benzil was studied, employing C,N- and N,N-nucleophiles, such as ethyl(3-amino substituted) 2-butenoates 4a-c , (S,Z)-ethyl 3-(1-ethoxy-3-hydroxy-1-oxopropan2-ylamino)but-2-enoate, semicarbazide or thiosemicarbazide 10, to evaluate their electrophilic centers as building blocks for the synthesis of the polyfunctionalized heterocyclic compounds, resulting in pyrrolinone 5a-c, pyrrole, triazinone and triazinethione. The employed benzil was obtained by the oxidation of the benzoin under solvent free conditions in a comparative study between different protocols of oxidation, using the methodology under mild reaction conditions and supported reagent associated to the microwave irradiation, with good results and without aggressive reagents[1].
Reference
1 Slaga T J, Klein-Szanto A J P, Triplett L L, et al. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound[J]. Science, 1981, 213(4511): 1023-1025.
2 Brown, C. J., and R. Sadanaga. "The crystal structure of benzil." Acta Crystallographica 18.2 (1965): 158-164.
You may like
Related articles And Qustion
Lastest Price from Benzil manufacturers

US $0.00/G/KG2025-04-23
- CAS:
- 134-81-6
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 100000000KG

US $1.00/Kg/Bag2025-04-21
- CAS:
- 134-81-6
- Min. Order:
- 1KG
- Purity:
- 98.0%min
- Supply Ability:
- 5ton/month