Benzil: A Versatile Reagent in Organic Chemistry and Beyond
Introduction to benzoin
Benzoin, also known as benzophenone, is a widely used organic compound. The state is a yellow crystalline solid. Due to its unique chemical properties and wide applications, especially in biphenyl compounds, Benzil is an important structural unit widely present in pharmaceutical compounds, pesticides, functional materials, etc. The synthesis of this structural block is crucial for the production of drugs, materials, and other materials. It is used as an organic synthesis intermediate, insecticide, photosensitive adhesive, UV curing coating, and UV curing agent for pharmaceutical intermediates.

Figure 1 Characteristics of Benzil
The Structure and Properties of Benzoin
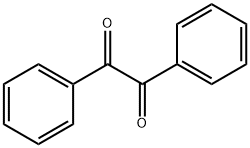
Benzoin is an aromatic diketone compound, and the molecular structure of Benzil contains two ketone groups (C=O) and two benzene rings. Two ketone groups are connected by a carbon carbon single bond, forming a highly symmetrical molecular structure. This symmetry gives benzoin stable chemical properties, but it also affects the physical properties such as melting point and solubility of Benzil.
In terms of physical properties, benzoin appears as yellow needle shaped crystals, with a melting point of approximately 94 ° C and a boiling point of 346 ° C. Benzil is insoluble in water, but soluble in organic solvents such as ethanol, ether, and benzene. In addition, due to the presence of conjugated double bond systems in benzoin molecules, they exhibit excellent optical activity and fluorescence properties.
The synthesis method of benzoin
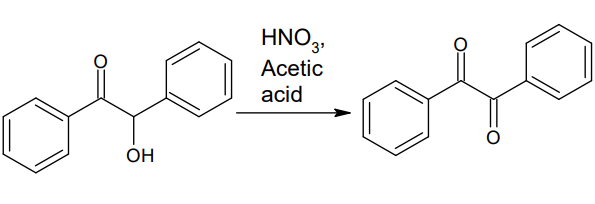
The most common synthesis method of benzimidazole is achieved through the oxidation reaction of benzoin. This reaction can be carried out in the laboratory using nitric acid, peroxides, or metal catalysts.
The synthesis steps of Benzil are as follows: first, using benzaldehyde as the raw material, benzoin (C14H12O) is prepared through benzoin condensation reaction.
Afterwards, benzoin is oxidized to form benzoin. Nitric acid or hydrogen peroxide are commonly used as oxidants, with relatively mild reaction conditions and high reaction efficiency.
This method has a clear reaction pathway and high yield, therefore it has been widely used in laboratories and industrial production. In addition, in recent years, some green chemistry methods have emerged, such as using photocatalysts or electrochemical methods to synthesize benzoin, further reducing environmental pollution.
Benzil Applications
Organic synthesis intermediates
Benzoin is widely used in organic synthesis, especially in the preparation of heterocyclic compounds. For example, it can react with compounds such as urea and aniline to produce oxazole and imidazole compounds, which have important pharmacological activities and have been widely studied and developed in medicinal chemistry.
Polymer and Materials Science
Due to its excellent photosensitivity and fluorescence properties, benzoin is widely used in the preparation of photoinitiators. Under the action of ultraviolet light, benzoin can trigger free radical polymerization reactions, which are particularly important in photolithography technology and polymer material manufacturing.
Optical Research and Analytical Chemistry
The fluorescence properties of benzoin make it a commonly used reagent in optical experiments and material detection. For example, it can be used to detect the absorption or emission of light of specific wavelengths. In addition, Benzil is also used to develop novel fluorescent probes and sensors.
Medical research
Benzoyl derivatives also have certain biological activities, especially in the development of antibacterial and anti-tumor drugs, showing broad application prospects. Through molecular modification, benzoin can be designed into a series of drug lead compounds with specific functions.
Benzil Security Information
Benzoin may irritate the skin, eyes, and respiratory tract, and should be washed immediately after contact.
When using and storing benzoin, care should be taken to avoid contact with oxidants.
When operating Benzil, it must be done in a well ventilated environment and maintain good personal protective measures, such as wearing chemical goggles and gloves.
If accidents such as inhalation, ingestion, or skin contact occur, please seek medical attention immediately and bring Benzil's relevant safety data to the hospital.
![Article illustration]() Reference
Reference
[1] Weiss M, Appel M. The catalytic oxidation of benzoin to benzil[J]. Journal of the American Chemical Society, 1948, 70(11): 3666-3667.
[2] Bera S C, Mukherjee R, Chowdhury M. Spectra of benzil[J]. The Journal of Chemical Physics, 1969, 51(2): 754-761.
Related articles And Qustion
See also
Lastest Price from Benzil manufacturers

US $0.00/G/KG2025-04-23
- CAS:
- 134-81-6
- Min. Order:
- 1G/KG
- Purity:
- 99%
- Supply Ability:
- 100000000KG

US $1.00/Kg/Bag2025-04-21
- CAS:
- 134-81-6
- Min. Order:
- 1KG
- Purity:
- 98.0%min
- Supply Ability:
- 5ton/month