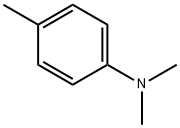
N,N-Dimethyl-p-toluidine, a Component in Dental Materials
Introduction
N,N-Dimethyl-p-toluidine is on the U.S. Environmental Protection Agency High Production Volume Chemical List with annual production estimates of 1 to 10 million pounds. DMPT is found in a wide variety of materials, and a recent search of U.S. patents indicates that there are over 900 patents that describe products containing DMPT. DMPT has been used in the preparation of acrylic bone cements and dental materials for over 50 years. DMPT is an accelerator in the redox initiator–accelerator system used commercially to cure methyl methacrylate monomers, but polymerization is rarely complete. Thus, there is potential for DMPT exposure to surgical staff, dental prosthetic device manufacturers, and users of DMTP-containing medical devices. Toxicity to DMPT has been reported in children after accidental DMPT poisoning accompanied by methemoglobinemia. Allergic responses to DMPT have also been reported. Medical devices, such as dental materials containing N,N-DIMETHYL-P-TOLUIDINE, are regulated under the 501(k) clearance process established by the U.S. Congress in 1976. [1]

Synthesis procedure
The lignin is reacted as a methyl source with an amine compound to prepare an N,N-Dimethyl-p-toluidine in a 16 ml PTFE-lined stainless steel reactor. A magnetic stirrer in the reactor. In the experiment, a certain amount of iodide catalyst and optionally a fluoroborate promoter, lignin or anisole. The amine and the reaction solvent are added to the reaction vessel, The reaction kettle was sealed and replaced three times with 1 MPa of nitrogen. The reactor is placed in an air bath at a constant temperature and charged. The magnetic stirring speed was set to 800 rpm. After the reaction, the reaction vessel was placed in an ice water bath to be cooled. About 0.04 g of 1,3,5-trioxane was added as an internal standard to the liquid mixture after the reaction. The liquid mixture was then diluted with 10 ml of methanol and stirred for 5 minutes. The mixture was centrifuged and subjected to 1H NMR (Bruker Avance III 400HD). The tape reagent used was DMSO-d6.The selectivity of the N,N-Dimethyl-p-toluidine product can reach nearly 100% under optimal reaction conditions. The yield of N,N-Dimethyl-p-toluidine was 72%.[2]
Toxic and carcinogenic effects
N,N-Dimethyl-p-toluidine induced toxic and/or carcinogenic effects in the hematologic system, liver, nasal cavity, and other organ systems in rats and/or mice. The hematologic toxicity was characterized by an increase in methemoglobin levels and a macrocytic, hypochromic, responsive anemia particularly in rats. The anemia was defined as an insufficient concentration of hemoglobin and erythrocytes larger than their normal volume. The mechanism for the anemia is thought to involve oxidative damage to hemoglobin leading to Heinz body formation and decreased erythrocyte survival. This effect is similar to methemoglobin-induced anemias in animals and humans after exposure to aniline and other nitroaromatic compounds.[3]
Methemoglobinemia has been observed in children ingesting artificial fingernail solutions containing N,N-Dimethyl-p-toluidine. This effect is thought to arise primarily through formation of p-methylphenylhydroxylamine. A similar metabolite of aniline, phenylhydroxylamine, induces methemoglobinemia in rats. Formation of p-methylphenylhydroxylamine may be inferred from the presence of p-(N-acetylhydroxyamino) hippuric acid in urine of N,N-Dimethyl-p-toluidine-treated rats. It could be speculated that N,N-Dimethyl-p-toluidine-N-oxide, also detected in urine of rats, contributes to methemoglobinemia. Dimethylaniline N-oxide has been shown to autocatalyze oxidization of hemoglobin. Further, methemoglobinemia has been attributed to formation of an N-oxide metabolite in humans ingesting toxic doses of the drug, zopiclone.
In mice, the hematologic toxicity was less severe than in rats, and while methemoglobin and Heinz body formation occurred, consistent decreases in the circulating erythroid mass or erythron change were not evident. The lower methemoglobin levels in mice may be due in part to a higher methemoglobin reductase activity than in rats or may occur as the result of quantitative differences in N,N-Dimethyl-p-toluidine metabolism between the two species. Mice receiving N,N-Dimethyl-p-toluidine by gavage excreted less p-(N-acetylhydroxyamino)hippuric acid in urine (as percentage total dose) than rats, indicating less potential formation of p-methylphenylhydroxylamine (unpublished NTP findings). Methemoglobin formation by oxidation of the heme moiety may be a sentinel response for N,N-Dimethyl-p-toluidine oxidative damage that eventually resulted in carcinogenic responses in liver, thyroid, lung, or forestomach. The occurrence of N,N-Dimethyl-p-toluidine-induced malignant liver neoplasms in rats and mice provided clear evidence for N,N-Dimethyl-p-toluidine carcinogenic activity. In rats, this included increases in hepatocellular carcinoma and hepatocellular adenoma or carcinoma (combined). In mice, this included increases in hepatocellular adenoma (increases in multiple hepatocellular adenoma in males and increases in multiple and total incidences of hepatocellular adenoma in females); increases in multiple and the total incidences of hepatocellular carcinoma in males and females; increases in hepatoblastoma in males and females; and increases in hepatocellular adenoma, hepatocellular carcinoma, or hepatoblastoma (combined) in males and females. Hepatocellular adenoma, hepatocellular carcinoma, and hepatoblastoma represent a biological and morphological continuum. Hepatoblastomas are uncommon spontaneous neoplasms that may occur after chemical administration (primarily in mice) and have previously been seen in NTP rodent studies of benzofuran, ethylene thiourea, o-nitroanisole, coumarin, methylphenidate hydrochloride, 1-amino-2,4-dibromoanthraquinone, oxazepam, pyridine, primidone, and goldenseal. Theymay arise from hepatocellular adenomas or carcinomas, and when this occurs, only the hepatoblastoma is recorded. Hepatoblastomas in humans account for approximately 70% of childhood liver cancers.
Multiple Aspects of Its Activity in Human Submandibular
In a human submandibular gland adenocarcinoma cell line with visible light irradiation, the photosensitizer camphorquinone in the presence of N,N-Dimethyl-p-toluidine demonstrated both dose- and time-dependent N,N-Dimethyl-p-toluidine induction of reactive oxygen species. This ability of N,N-Dimethyl-p-toluidine to form free radicals with subsequent DNA damage may explain the N,N-Dimethyl-p-toluidine carcinogenic mechanism. N,N-Dimethyl-p-toluidine has been reported to have estrogen antagonist activity in vitro , but whether this activity contributes to N,N-Dimethyl-p-toluidine carcinogenic activity is not known. The N,N-Dimethyl-p-toluidine-induced nasal and pulmonary toxic lesions are not typical of gavage-associated injury or aspiration. The N,N-Dimethyl-p-toluidine respiratory epithelial degeneration/necrosis may be due to cytotoxicity as a result of pulmonary/nasal epithelial cytochrome P450 metabolic activation resulting in production of toxic N,N-Dimethyl-p-toluidine metabolites. Metabolic activation of specific P450 enzymes such as CYP2EI and CYP2A5 are reported to result in olfactory epithelial damage secondary to orally administered acetaminophen to mice.
N,N-Dimethyl-p-toluidine belongs to the N-dialkylaminoaromatic class of compounds, with an aromatic amine structural alert. Carcinogenic arylamines undergo metabolic activation via N-hydroxylation followed by O-esterification. Members of the alkylaniline class of compounds, including p-toluidine (4-methylaniline), may form DNA adducts via this mechanism. It is possible that p-methylphenylhydroxyamine is formed from N,N-Dimethyl-p-toluidine leading to DNA adduct formation, especially at high doses. Additionally, the p-alkylaniline structural alert for N,N-Dimethyl-p-toluidine indicates potential formation of a reactive imine methide. Electrophilic imine methides, functioning as nitrogen/carbon-centered free radicals, are capable of damaging DNA or proteins. An imine methide is thought to be involved in pulmonary toxicity of 3-methylindole and hepatotoxicity of diclofenac. Further, correlated covalent binding to plasma proteins in humans with an imine methide metabolite of the drug, Eltrombopag. N,N-Dimethyl-p-toluidine toxicity correlated with the level of N,N-Dimethyl-p-toluidine deposition in N,N-Dimethyl-p-toluidine target organs. Dix reported that the highest concentrations of 14C-N,N-Dimethyl-p-toluidine occurred in liver and kidney of rats following oral N,N-Dimethyl-p-toluidine exposure. Similar N,N-Dimethyl-p-toluidine exposures in mice resulted in high N,N-Dimethyl-p-toluidine levels in the liver and lung. The N,N-Dimethyl-p-toluidine toxicity observed in these rodent studies is consistent with findings in humans where oral N,N-Dimethyl-p-toluidine exposure caused acute cyanosis due to methemoglobinemia formation.
References
[1] Atsumi T, Iwakura I, Fujisawa S, Ueha T. The production of reactive oxygen species by irradiated camphorquinone-related photosensitizers and their effect on cytotoxicity. Arch Oral Biol. 2001;46:391–401. doi: 10.1016/s0003-9969(01)00005-x.
[2] INSTITUTE OF CHEMISTRY OF CHINESE ACADEMY OF SCIENCES - CN109776302, 2019, A
[3] Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–31.
See also
Lastest Price from N,N-Dimethyl-p-toluidine manufacturers

US $20.00-15.00/kg2025-08-07
- CAS:
- 99-97-8
- Min. Order:
- 1000kg
- Purity:
- 99%
- Supply Ability:
- 200 mt

US $6.00/kg2025-04-21
- CAS:
- 99-97-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month