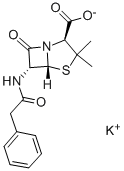
Penicillin G potassium salt: a normal form of penicillin
Introduction
Penicillin G potassium salt is a widely studied form of penicillin used extensively in microbiological research for its antibacterial activity. Researchers utilize this compound to investigate the mechanisms of bacterial growth inhibition, particularly in gram-positive organisms, where it disrupts cell wall synthesis. In molecular biology, it serves as a selective agent to maintain the growth of penicillin-resistant strains of bacteria or to ensure the purity of bacterial cultures by eliminating contaminating susceptible strains. This potassium salt form offers enhanced solubility, making it suitable for use in various aqueous media, including growth media and experimental buffers. Additionally, its role in the study of bacterial resistance mechanisms is crucial, as it helps in understanding how resistance genes are transferred and expressed within microbial communities.[1]

Penicillin G was first crystallized at Squibb by MacPhillamy, Wintersteiner and Alicino, where the existence of sulfur in the molecule was first noted by Alicino due to its characteristic odor. The structure of benzylpenicillin was established by Dorothy Hodgkin and Barbara Lois in May 1945, using three-dimensional x-ray crystallography. Thus, it was shown that penicillin contained a previously unknown ring system. Penicillin G Potassium salt is the United States adopted name. Penicillin G Potassium salt is a white, free flowing crystalline powder with a faint pungent odor characteristic of penicillins. The compound itself should not be mixed with acids, alcohol, bases, ephredrine, glycerol, iodine and iodides, naphthalene oils, oxidizing agents, resorcinol, salts of heavy metals, vitamin B, and zinc oxide. The significant adverse reaction to benzylpenicillin is the induction of an allergic reaction as severe as anaphylactic shock leading to death.
Synthesis
A series of experiments was carried out in which to 1000 ml of an activated- carbon-treated extract containing approximately 100,000 Oxford Units Penicillin G per ml in n-butylacetate, a 50% (w/w) solution of K2CO3 in water was added such that the mixture contained approximately 1.0 mole-equivalent of potassium with regard to penicillin G. The resulting mixture was heated under vacuum using a rotary film evaporator (bath 45-489C) whereby a certain quantity of the mixture was evaporated. Subsequently, methanol was added to the residue and the mixture was agitated during 1 h at 25^. The suspension was filtered and the wet cake was washed with 70 ml n-butylacetate after which the wet cake was dried. The volume evaporated is the total volume evaporated and the value in brackets represents the volume of the water layer in the distillate. The column with the water content represents the water content of the residue after evaporation. All experiments yielded white Penicillin G potassium salt crystals in high yield (as defined hereinbefore) with regard to the penicillin content in the carbon treated extract (=100%) and only limited penicillin losses to the mother liquor. The crystals from experiment 1 contained 99.3% Penicillin G potassium salt (i.e. good quality).[2]
CLINICAL PHARMACOLOGY
Aqueous Penicillin G potassium salt is rapidly absorbed following both intramuscular and subcutaneous injection. Initial blood levels following parenteral administration are high but transient. Penicillins bind to serum proteins, mainly albumin. Therapeutic levels of the penicillins are easily achieved under normal circumstances in extracellular fluid and most other body tissues. Penicillins are distributed in varying degrees into pleural, pericardial, peritoneal, ascitic, synovial, and interstitial fluids. Penicillins are excreted in breast milk. Penetration into the cerebrospinal fluid, eyes, and prostate is poor. Penicillins are rapidly excreted in the urine by glomerular filtration and active tubular secretion, primarily as unchanged drug. Approximately 60 percent of the total dose of 300,000 units is excreted in the urine within this 5-hour period. For this reason, high and frequent doses are required to maintain the elevated serum levels desirable in treating certain severe infections in individuals with normal kidney function. In neonates and young infants, and in individuals with impaired kidney function, excretion is considerably delayed.
After an intravenous infusion of Penicillin G potassium salt, peak serum concentrations are attained immediately after completion of the infusion. In a study of ten patients administered a single 5 million unit dose of Penicillin G potassium salt intravenously over 3 to 5 minutes, the mean serum concentrations were 400 mcg/mL, 273 mcg/mL and 3 mcg/mL at 5 to 6 minutes, 10 minutes and 4 hours after completion of the injection, respectively. In a separate study, five healthy adults were administered one million units of Penicillin G potassium salt intravenously, either as a bolus over 4 minutes or as an infusion over 60 minutes. The mean serum concentration eight minutes after completion of the bolus was 45 mcg/mL and eight minutes after completion of the infusion was 14.4 mcg/mL. The mean β-phase serum half-life of Penicillin G potassium salt administered by the intravenous route in ten patients with normal renal function was 42 minutes, with a range of 31 to 50 minutes. The clearance of Penicillin G potassium salt in normal individuals is predominantly via the kidney. The renal clearance, which is extremely rapid, is the result of glomerular filtration and active tubular transport, with the latter route predominating. Urinary recovery is reported to be 58 to 85% of the administered dose. Renal clearance of penicillin is delayed in premature infants, neonates and in the elderly due to decreased renal function. The serum half-life of Penicillin G potassium salt correlates inversely with age and clearance of creatinine and ranges from 3.2 hours in infants 0 to 6 days of age to 1.4 hours in infants 14 days of age or older. Nonrenal clearance includes hepatic metabolism and, to a lesser extent, biliary excretion. The latter routes become more important with renal impairment. Probenecid blocks the renal tubular secretion of penicillin. Therefore, the concurrent administration of probenecid prolongs the elimination of Penicillin G potassium salt and, consequently, increases the serum concentrations. Penicillin G potassium salt is distributed to most areas of the body including lung, liver, kidney, muscle, bone and placenta. In the presence of inflammation, levels of penicillin in abscesses, middle ear, pleural, peritoneal and synovial fluids are sufficient to inhibit most susceptible bacteria. Penetration into the eye, brain, cerebrospinal fluid (CSF) or prostate is poor in the absence of inflammation. With inflamed meninges, the penetration of Penicillin G potassium salt into the CSF improves, such that the CSF/serum ratio is 2 to 6%. Inflammation also enhances its penetration into the pericardial fluid. Penicillin G potassium salt is actively secreted into the bile resulting in levels at least 10 times those achieved simultaneously in serum.[3]
![Article illustration]()
![Article illustration]() Microbiology
Microbiology
Penicillin G potassium salt is bactericidal against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall peptidoglycan rendering the cell wall osmotically unstable. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci or against organisms resistant to beta-lactams because of alterations in the penicillin-binding proteins, such as methicillin-resistant staphylococci. Penicillin G potassium salt is highly active in vitro against streptococci (groups A, B, C, G, H, L, and M) and Neisseria meningitidis.
Other organisms susceptible in vitro to Penicillin G potassium salt are Neisseria gonorrhoeae,Corynebacterium diphtheriae, Bacillus anthracis, clostridia, Actinomyces species, Spirillum minus, Streptobacillus moniliformis, Listeria monocytogenes, and leptospira;Treponema pallidum is extremely susceptible. Some species of gram-negative bacilli were previously considered susceptible to very high intravenous doses of Penicillin G potassium salt (up to 80 million units/day) including some strains of Escherichia coli, Proteus mirabilis, salmonella, shigella, Enterobacter aerogenes (formerly Aerobacter aerogenes) and Alcaligenes faecalis. Penicillin G potassium salt is no longer considered a drug of choice for infections caused by these organisms.
References
[1] Kambe, Y. (1978). Growth and cell structure of oral spirochetes and effect of benzyl penicillin potassium (author's transl). Shigaku = Odontology; Journal of Nihon Dental College, 66(1), 30-70.
[2] DSM SINOCHEM PHARMACEUTICALS NETHERLANDS - WO2007/63107, 2007, A1 Location in patent: Page/Page column 7-8
[3] Li, K., Mohammed, M. A. A., & Liu, Z. (2020). Recent progress in the development of immobilized penicillin G acylase for chemical and industrial applications: A mini-review. Polymers for Advanced Technologies, 31(3), 368-388.
You may like
See also
Lastest Price from Penicillin G potassium salt manufacturers

US $1.00/KG2025-04-21
- CAS:
- 113-98-4
- Min. Order:
- 1KG
- Purity:
- ≥96.0%
- Supply Ability:
- 1000kg/month

US $1.00/KG2025-04-21
- CAS:
- 113-98-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt