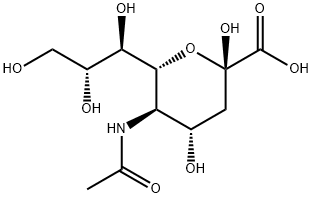
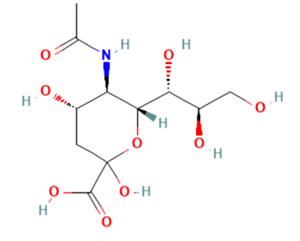
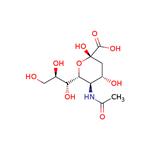
N-Acetylneuraminic Acid: A Comprehensive Overview for Chemistry Professionals
N-acetylneuraminic acid is a derivative of 9-carbon monosaccharide. It is a negatively charged ion that makes saliva feel smooth. It is a component of the brain and can help children's brain development and retinal development. It was first extracted and separated from salivary gland mucin by Swedish biochemist Gunmar Blix, hence the name sialic acid.

Figure 1 Characteristics of N-Acetylneuraminic acid
What is N-acetylneuraminic acid?
N-acetylneuraminic acid is an essential nutrient for the human body. After intake, it can effectively enhance human immunity, fight free radicals, and delay skin aging. Sialic acid also has a certain antiviral effect and can prevent colds. Sialic acid can accelerate the heart's ejection capacity and has a certain regulating effect on palpitations. In addition, the collagen content in sialic acid is relatively sufficient, which can increase skin elasticity and eliminate fine facial wrinkles.
In 2016, the US FDA approved N-acetylneuraminic acid for infant formula and ordinary food (GRAS certification). In 2017, the European Food Safety Authority approved sialic acid as a new food ingredient. The domestic N-acetylneuraminic acid (sialic acid) new food raw material was applied by Zhongke Hongji Biotechnology Co., Ltd. in 2012 and passed the review of the National Health and Family Planning Commission on May 31, 2017. It does not specify unsuitable groups and can be used in infant formula milk powder.
Distribution of N-acetylneuraminic acid in the human body
N-acetylneuraminic acid is an important nutrient in natural milk. The content of N-acetylneuraminic acid in the human brain is 20 times that of other cells. It is an essential nutrient for brain cognitive development. The content of sialic acid in human brain nerve tissue is the highest. The content of sialic acid in gray matter is 15 times that of internal organs such as liver and lungs. The content of sialic acid in human brain cells is 2-4 times that of other animals. This is why sialic acid is also called neuraminic acid.
Important role of N-acetylneuraminic acid
1. Promote brain and retinal development
N-acetylneuraminic acid is known as "brain gold". It is the main component of neurons and nerve fibers and can promote the development of fetal brain and retina.
2. Improve the intelligence and memory of infants and young children
Sialic acid is an important component of brain gangliosides and a brain nutrient. It acts on brain cell membranes and synapses, and can promote the speed of information transmission of brain nerve cells, thereby promoting the development of memory and intelligence of infants and young children.
3. Anti-Alzheimer's disease
N-acetylneuraminic acid participates in the metabolic process of nerve cells, protects and stabilizes nerve cells, and thus can play an anti-Alzheimer's disease role.
4. Improve human immunity
Sialic acid will not be degraded by digestive enzymes in the digestive system, and can enter the intestines, preventing pathogenic microorganisms from adsorbing to intestinal cells and resisting the invasion of various pathogens. Therefore, it can play a role in improving human immunity. In addition, sialic acid can also improve the intestinal absorption capacity of minerals and vitamins, and enhance the body's absorption level of nutrients.
5. N-acetylneuraminic acid has antiviral effects.
Sialic acid is a receptor for influenza viruses, which helps the human body defend and eliminate influenza viruses as soon as possible.
6. Improve dry eyes and eye fatigue.
You may like
Related articles And Qustion
See also
Lastest Price from N-Acetylneuraminic acid manufacturers
US $990.00/g2025-11-24
- CAS:
- 131-48-6
- Min. Order:
- 1g
- Purity:
- 99 %
- Supply Ability:
- 500 Kg

US $0.00/kg2025-07-18
- CAS:
- 131-48-6
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 500KGS