Physiological Function and Preparation of N-Acetylneuraminic acid
General description
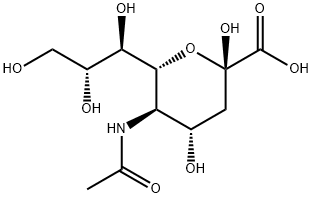
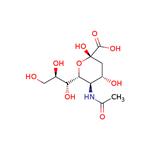
N-acetylneuraminic acid (NeuAc), also known as sialic acid, is an acetylated derivative of acid sugar neuraminic acid [1]. CAS No: 131-48-6. Its chemical name is 5-amino-3,5-dideoxy-d-glycerol-d-galactonose (Figure 1).
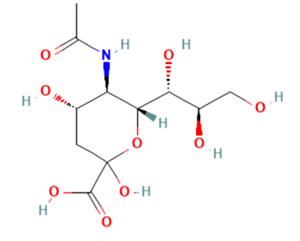
Figure 1. The molecular formula of N-acetylneuraminic acid
NeuAc is widely present in mammalian tissues. It is a component of oligosaccharide chains of mucins, glycoproteins and glycolipids, occupies the terminal non-reducing positions of complex carbohydrate oligosaccharide chains, and is attached to the inner and outer surfaces of cell membranes in various ways, which plays an important role in many physiological and immune recognition processes, such as cell adhesion, glycoprotein stability, cytotoxicity, and tumor metastasis, and has attracted more and more attention [2].
Physiological Function of N-acetylneuraminic acid
NeuAc, which exists on the surface of cell membrane, has a negative charge, is the main source of negative charge of cell membrane, and is closely related to cell adhesion. It has the Physiological functions of anti recognition, improving infant intelligence and memory, anti senile dementia, improving intestinal absorption of vitamins and minerals, anti-virus, antibacterial and detoxification [3-6].
Nervous system and brain development
NeuAc is essential for the development and function of the nervous system. It is involved in regulating synaptogenesis, neurogenesis, cell proliferation and migration, cell adhesion and axon guidance, and regulates innate immune function, especially NeuAc, which plays an important role in learning and memory. Dietary supplement of NeuAc can increase the content of NeuAc in the brain to improve learning and memory. Studies have shown that NeuAc supplementation in the diet is beneficial to infant bone growth, brain development and the maintenance of brain function in the elderly. In addition, the content of NeuAc in the brain of patients with Alzheimer's disease and schizophrenia decreased. After NeuAc drug treatment, the content of NeuAc returned to normal, which shows that NeuAc is closely related to neural activity.
Reproductive and immune system functions
In the human reproductive system, NeuAc plays an important physiological function during the maturation and fertilization of sperm and egg cells, the contact between sperm and egg, and the interaction between sperm and various liquids and surfaces of the female reproductive tract before reaching the egg. It is essential to protect the extraembryonic tissues of the fetus from maternal complement attack. Fully sialized glycoproteins and cells are complete and tolerant by organisms. Once NeuAc is lost, molecules and cells become "non-self", no longer adapt to the chemical structure required for normal "behavior", and are recognized and destroyed by the organism's defense system. This defense system is part of the immune system, so NeuAc plays a crucial role in immunology.
Tumor
In tumor cells, the masking effect of NeuAc is also of great significance. The high coating of tumor cells by NeuAc often accelerates cancer progression, promotes immune escape, enhances tumor proliferation and metastasis, helps tumor angiogenesis, and helps resist apoptosis and cancer therapy. The alteration of NeuAc on the cell surface is caused by sialyltransferase, and the uridine and hypoxanthine derivatives of NeuAc are strong inhibitors of sialyltransferase, which can inhibit cancer cell metastasis.
Antioxidant
The active hydroxyl group of NeuAc can provide active hydrogen to combine with superoxide anion radical (O2-) and hydroxyl radical (-OH) to resist free radical oxidation. It should be noted that only NeuAc in free state can play an antioxidant role. In addition, hydroxyl can also complex with metal ions to reduce the production of free radicals. In vitro experiments show that NeuAc in bird's nest can effectively inhibit the activity of tyrosine hydroxylase, reduce the production of polyamine, prevent the formation of melanin, and have a certain whitening effect.
Other functions
The N-acetylneuraminic acid can enhance intestinal absorption of minerals and vitamins and promote bone development. Moreover, NeuAc peptides can prevent intestinal toxins and pathogenic bacteria from binding to intestinal mucosal cells, thereby improving the antibacterial, detoxifying and antiviral capabilities of the intestine. Since NeuAc adheres to the surface of red blood cells, it can protect red blood cells and prevent unnecessary cell interactions in blood circulation, so it is also critical in regulating the longevity of these blood components and maintaining homeostasis. In addition, it can be used to treat rheumatoid arthritis, because NeuAc on the cell surface can effectively prevent the excessive accumulation of white blood cells and play an anti-inflammatory role.
Preparation
The preparation methods of N-acetylneuraminic acid mainly include extraction from natural products, chemical synthesis, polymer degradation, enzymatic synthesis, whole cell catalysis and microbial fermentation [7-10].
Extract from natural products
Sialic acid existing on the cell surface is mainly in the form of glycoconjugates, but the content is extremely low. The traditional method is to extract natural products such as cubilose, eggs and milk. However, extraction of NeuAc from natural raw materials has many disadvantages, including difficulties in obtaining materials, complex extraction processes, low NeuAc content, low recovery rate, and a requirement for specific equipment. Therefore, extraction of NeuAc from natural raw materials struggles to meet the demand for industrial-scale production of NeuAc.
Chemical synthesis
NeuAc was obtained by propenylation and ozonolysis of ManNAc with α-bromomethacrylic acid in an acidic alcohol solution using indium as a catalyst. The conditions for the synthesis of NeuAc by chemical synthesis are relatively strict. In addition to the need for toxic rare metals such as indium as catalysts, the product recovery rate is not high, so it is difficult to be widely used.
Enzymatic synthesis
The first step of enzymatic synthesis of NeuAc is to isomerize GlcNAc to ManNAc through the action of n-acetyl-d-glucosamine-2-epiisomerase (AGE). Then ManNAc and pyruvate condense to form NeuAc under the action of N-acetylneuraminate lyase (NanA). AGE and NanA are required in these two steps; Or NeuAc can be directly produced with ManNAc as the substrate, as shown in Figure 2.
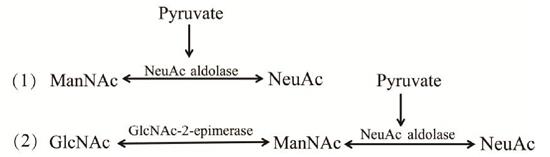
Figure 2. Two methods for enzymatic synthesis of NeuAc
Although the enzymatic synthesis of NeuAc has the advantages of high conversion, high purity and easy extraction and separation, the enzymes needed in the experiment must be purified, the steps are cumbersome and time-consuming, and ATP for activating age must be added. When NanA is not easy to obtain, the large-scale production of enzymatic synthesis is also limited accordingly.
Whole-cell biocatalysis
Whole-cell biocatalysis plays an important role in the large-scale synthesis of agrochemical intermediates, chemicals, and food additives, which can avoid lengthy enzyme purification steps and reduce production costs. Efficient whole-cell biocatalytic process for the synthesis of NeuAc using N-acetylneuraminic acid lyase with GlcNAc or ManNAc as substrates with yields as high as 18.32 g L-1. Due to the need to add toxic inducers in the whole-cell catalysis process, coupled with the obstruction of cell membranes and the interference of side reactions, the realization of large-scale production requires a combination of biocatalysis and chemical catalysis to a certain extent.
Microbial fermentation
The microbial fermentation method can directly use glucose, glycerol or other carbon sources as substrates without adding any other precursors. Escherichia coli fermentation to synthesize polysialic acid and then hydrolyze it to prepare NeuAc is the only method currently approved for the production of NeuAc in China. Few types of bacterial cells in nature can produce polysialic acid, and Escherichia coli is one of them. E.coli K1 and E.coli K235 are usually selected, and sorbitol, glucose or xylose are used as substrates. Microbial fermentation plays an important role in the production of NeuAc due to its relatively low production cost and wide research field.
Metabolism[11]
In vivo, the liver is the main synthetic organ of NeuAc. In the cytoplasm, NeuAc is produced by a long way from glucose (Figure 3). Sialylation occurs in the cytoplasm, in which a variety of molecules are involved. The free NeuAc residues are phosphorylated, which are pumped into the Golgi cavity in the form of CMP-NeuAc via reverse transporters. After being modified by methylation, lactosylation, acetylation, etc., sialyltransferase is connected to the sugar chain to form sialic acid complexes with different functions. This process is called "sialylation". NeuAc on the sugar chain is finally removed by sialidase in the body or lysosome, and free NeuAc is formed again, so as to achieve the purpose of recycling.
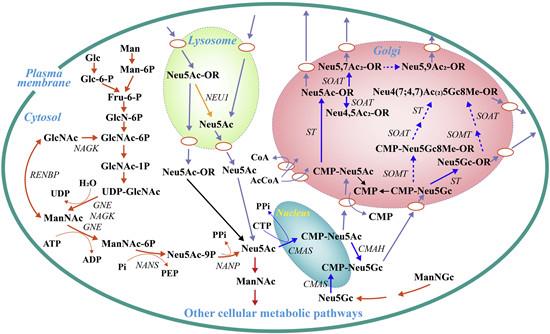
Figure 3. Metabolic pathway of N-acetylneuraminic acid in vivo
References
1. JC A, GK B, DIRS C, et al. Assays for the identification and quantification of sialic acids: Challenges, opportunities and future perspectives-science direct[J]. Bioorganic&Medicinal Chemistry, 2020.
2. WANG B, BRAND-MILLER J. The role and potential of sialic acid in human nutrition[J]. European Journal of Clinical Nutrition, 2003, 57(11):1351-1369.
3. YANG H Q, LU L P, CHEN X Z. An overview and future prospects of sialic acids[J]. Biotechnology Advances, 2021, 46:107678.
4. MAHAJAN V S, PILLAI S. Sialic acids and autoimmune disease[J]. Immunological Reviews, 2016, 269(1):145-161.
5. ZHOU, YANG, GUAN. Biological functions and analytical strategies of sialic acids in tumor[J]. Cells, 2020, 9(2):273.
6. WEIGEL P H, YIK J H. Glycans as endocytosis signals:The cases of the asialoglycoprotein and hyaluronan/chondroitin sulfate receptors[J]. Biochim Biophys Acta, 2002, 1572:341-363.
7. Xu P, Qiu J H, Zhang Y N, et al. Efficient Whole-Cell Biocatalytic Synthesis of N-Acetyl-D-neuraminic Acid[J]. Advanced Synthesis & Catalysis, 2007, 349(10): 1614-1618.
8. Samain. High yield production of sialic acid (Neu5Ac) by fermentation[J]. Canadian Patent, 02665332. 2009-04-03.
9. ZHAN X B, ZHU L, WU J R, et al. Production of polysialic acid from fed-batch fermentation with pH control[J].Biochem Eng J, 2002, 11:201-204.
10. Yang H, Lu L, Chen X. An overview and future prospects of sialic acids[J]. Biotechnology Advances, 2021, 46: 107678.
11. WANG B. Sialic acid is an essential nutrient for brain development and cognition[J]. Annual Review of Nutrition,2009, 29(1):177-222.
Related articles And Qustion
Lastest Price from N-Acetylneuraminic acid manufacturers
US $990.00/g2025-11-24
- CAS:
- 131-48-6
- Min. Order:
- 1g
- Purity:
- 99 %
- Supply Ability:
- 500 Kg

US $0.00/kg2025-07-18
- CAS:
- 131-48-6
- Min. Order:
- 1kg
- Purity:
- 98%min
- Supply Ability:
- 500KGS