MonoMethyl auristatin E: Application, Pharmacokinetics and Synthesis
General description
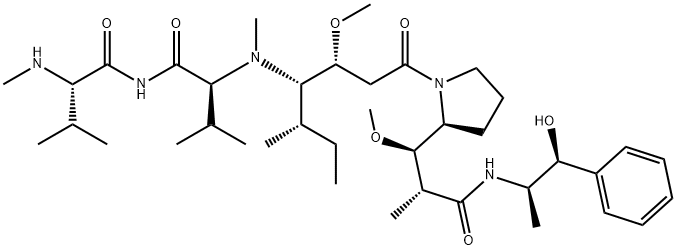
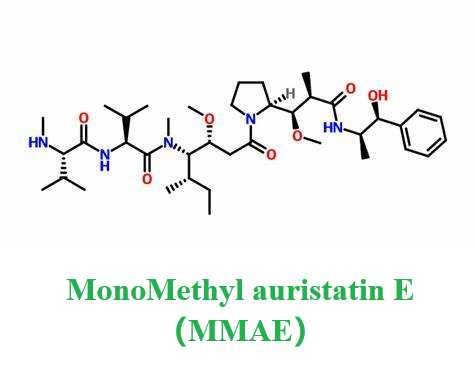
Monomethyl auristatin E is characterized as a linear depsipeptide consisting of five derivative amino acids with methyl and methoxy-substituted side chains. It is an anti-mitotic agent, which can inhibit cell division by blocking the polymerization of tubulin. Meanwhile, Monomethyl auristatin E is also a synthetic anti-tumor agent, which has attracted considerable attention in the field of antibody-coupled drugs (ADC). Because of its toxicity, it cannot be used as a drug itself, but is connected with monoclonal antibody (MAB). Besides, it is also an effective anti-mitotic drug derived from a polypeptide called dolastatin that exists in the marine shellless mollusk Dolabella auricularia. These peptides have shown effective in vitro and in vivo activities against a variety of lymphomas, leukemias and solid tumors in preclinical studies. The efficacy of these drugs is up to 200 times that of vinblastine. Its appearance is as follows:

Figure 1 Appearance of MonoMethyl auristatin E.
Chemical synthesis
MonoMethyl auristatin E can be synthesized by 3 steps according to the previous work [1]. Step 1: 1200 mg (2.33 mmol) of N-(tert-butoxycarbonyl)-N-methyl-L-valyl-N-[(2R,3S,4S)-1-carboxy-2-methoxy-4-methylhexan-3-yl]-N-methyl-L-valinamide were added together with 910.8 mg (2.33 mmol) of benzyl (2R,3R)-3-methoxy-2-methyl-3-[(2S)-pyrrolidin-2-yl]propanoate trifluoroacetate, 1327 mg (3.49 mmol) of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate and 2027 μl of N,N-diisopropylethylamine in 50 ml of DMF, and the mixture was stirred at RT for 5 min. Thereafter, the solvent was removed in vacuum. The remaining residue was taken up in ethyl acetate and extracted by shaking it successively with 5% aqueous citric acid solution and saturated sodium hydrogencarbonate solution. The organic phase was removed and concentrateof theorye residue was purified by means of preparative HPLC. The product fractions were combined and concentrated, and the residue was dried under high vacuum. benzyl ester intermediate N-(tert-butoxycarbonyl)-N-methyl-L-valyl-N-[(3R,4S,5S)-1-{(2S)-2-[(1R,2R)-3-(benzyloxy)-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl}-3-methoxy-5-methyl-1-oxoheptan-4-yl]-N-methyl-L-valinamide as a resin. Yield 1000 mg (55% of theory). LC-MS (Method 1): Rt = 1.56 min; MS (ESIpos): m/z 775 (M+H)+.
Step 2: The entire amount of this intermediate obtained was added in 25 ml of a mixture of methanol and dichloromethane (20:1), and the benzyl ester group was removed by hydrogenation under standard hydrogen pressure with 10% palladium on activated carbon as a catalyst. After stirring at RT for 30 min, the catalyst was filtered and the filtrate was concentrated in vacuum. Yield 803 mg (91% of theory). white solid. HPLC: Rt = 2.1 min; LC-MS: Rt = 1.24 min; MS (ESIpos): m/z = 685 (M+H)+.
Step 3: 50 mg (70 μmol) of intermediate above and 11 mg (70 μmol) of (1S,2R)-2-amino-1-phenylpropan-1-ol in 10 ml of DMF were admixed with 42 mg (0.11 μmol) of O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate and 25 μl of N,N-diisopropylethylamine, and the reaction mixture was stirred at RT for 5 min. This was followed by concentration and purification of the residue by means of preparative HPLC. After combining the corresponding fractions, concentrating and drying under high vacuum, 49 mg (81%) of the protected intermediate were obtained. Subsequently, the Boc group was cleaved using known conditions with trifluoroacetic acid in dichloromethane. Concentration was followed by the purification of the title compound via preparative HPLC. Yield 26 mg (52%). HPLC: Rt = 1.65 min; LC-MS (Method 1): Rt = 0.77 min; MS (ESIpos): m/z = 718 (M+H)+.
Application
MonoMethyl auristatin E is a new type of drug that is synthesized from a highly toxic polypeptide extracted from sea rabbits in the Indian Ocean after being modified. It has a strong anti-tumor effect. It is an anti-tubule drug like vinblastine, but its toxicity is about 200 times that of vinblastine. It plays an effective mitotic inhibition role by inhibiting the polymerization of tubulin. It is widely used as a cytotoxic component to produce antibody-drug conjugates (ADCs) for the treatment of cancer [2]. MonoMethyl auristatin E is a synthetic derivative of dolastatin-10 developed in the lab of George Pettit [3]. Previous investigations have revealed that MonoMethyl auristatin E induces apoptosis through a mechanism of cell death called mitotic catastrophe [4]. After ADC internalization, the released MonoMethyl auristatin E in the tumor cells can enter surrounding cells and cause bystander killing.
Pharmacokinetics
While MonoMethyl auristatin E is rapidly eliminated from the plasma, it shows prolonged and extensive distribution in tissues, blood cells, and tumor. Highly perfused tissues (e.g., lung, kidney, heart, liver, and spleen) demonstrated tissue-to-plasma area under the concentration curve (AUC) ratios > 20, and poorly perfused tissues (e.g., fat, pancreas, skin, bone, and muscle) had ratios from 1.3 to 2.4. MonoMethyl auristatin E distribution was limited in the brain, and tumor had 8-fold higher exposure than plasma [5].
References
[1]Lerchen et al. Novel antibody-peptide drug conjugates (ADCs) and their use and preparation. From PCT Int. Appl., 2012143497, 26 Oct 2012.
[2]Machulkin et al. Synthesis, characterization, and preclinical evaluation of a small-molecule prostate-specific membrane antigen-targeted monomethyl auristatin E conjugate. Journal of Medicinal Chemistry, 2021, 64(23): 17123-17145.
[3]Pettit et al. Antineoplastic agents 337. Synthesis of dolastatin 10 structural modifications. Anticancer Drug Des. 1995; 10(7):529–544.
[4]Portugal et al. Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des. 2010; 16(1):69–78.
[5]Chang et al. Whole-Body Pharmacokinetics and Physiologically Based Pharmacokinetic Model for Monomethyl Auristatin E (MMAE). J. Clin. Med. 2021, 10(6), 1332.
You may like
Related articles And Qustion
Lastest Price from MonoMethyl auristatin E manufacturers

US $30.00/kg2023-09-07
- CAS:
- 474645-27-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000t/year
![474645-27-7 MMAE;[3H]-Vedotin](/ProductImageEN/2021-07/Small/fa121420-61aa-42c9-9636-39edf05c31a7.jpg)
US $15.00-10.00/KG2021-07-13
- CAS:
- 474645-27-7
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton