Monomethyl Auristatin E: Advancing Cancer Treatment through Antibody-Drug Conjugates
General Description
Monomethyl Auristatin E, a potent cytotoxic agent derived from Dolastatin 10, is instrumental in cancer treatment through its role in Antibody-Drug Conjugates. It operates by inhibiting tubulin polymerization, leading to cell cycle arrest and death by disrupting microtubule networks essential for mitosis. Specifically targeting cancer cells while sparing healthy ones, Monomethyl Auristatin E is linked to monoclonal antibodies that home in on tumor-specific antigens, such as CD30, facilitating targeted therapy with reduced systemic toxicity. However, its use has raised concerns about potential neurotoxicity, with increasing reports of peripheral neuropathy among patients treated with Monomethyl Auristatin E-containing ADCs. This underscores the importance of monitoring and managing its neurological side effects to optimize therapeutic efficacy and safety.

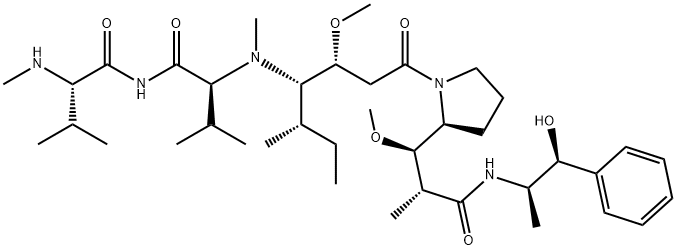
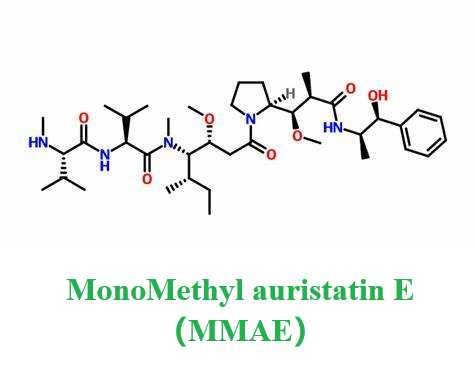
Figure 1. Monomethyl auristatin E
Overview
Monomethyl auristatin E is a potent cytotoxic agent used in the treatment of cancer, acting by killing cancer cells. Derived as an analog of Dolastatin 10, a microtubule inhibitor originally isolated from the sea hare Dolabella auricularia, Monomethyl auristatin E disrupts the cellular cytoskeleton and interferes with mitosis, leading to cell death. Microtubules, essential components of the cell's skeleton, are composed of alpha and beta tubulin proteins. Monomethyl auristatin E, along with its counterpart Monomethyl auristatin F (MMAF), is widely utilized as a payload component in Antibody-Drug Conjugates (ADCs), targeting specific cancer cells while minimizing the impact on healthy cells. The molecule comprises four amino acids: methylvaline (MeVal), valine (Val), dolaisoleuine (Dil), and dolaproine (Dap), ending in a carboxy-terminal phenylalanine replaced by aminomethylcoumarin in MMAF, which significantly reduces its cell activity. Monomethyl auristatin E is essentially a demethylated form of auristatin E, with only one methyl group at the N-terminus, making it significantly more potent than traditional chemotherapy agents like doxorubicin. Its high toxicity precludes its use as a standalone drug, but when linked to monoclonal antibodies that target specific markers on cancer cells, Monomethyl auristatin Ecan be delivered directly to the tumor site, enhancing therapeutic efficacy and reducing side effects. 1
Applications in Antibody-Drug Conjugates
Monomethyl Auristatin E is a potent cytotoxic agent widely utilized in Antibody-Drug Conjugates for treating various cancer types. Monomethyl Auristatin E acts by inhibiting tubulin polymerization, thereby disrupting the microtubule network essential for cell division and proliferation. This mechanism leads to cell cycle arrest at the G2/M phase and eventual cell death. In ADCs like Adcetris, Monomethyl Auristatin E is linked to a monoclonal antibody specific to a tumor-associated antigen, such as CD30 in the case of Adcetris, allowing targeted delivery of the cytotoxic agent to cancer cells while sparing healthy cells. Upon binding to the CD30 on the surface of tumor cells, the ADC is internalized, and Monomethyl Auristatin E is released within the cell through linker cleavage by lysosomal enzymes. The released MMAE then binds to microtubules, exerting its cytotoxic effect. This targeted approach enhances the therapeutic efficacy of Monomethyl Auristatin E while reducing systemic toxicity. However, the development of ADCs incorporating Monomethyl Auristatin E requires careful analysis and characterization to ensure safety, including studies on the pharmacokinetics of antibody-conjugated MMAE, total antibody, and free MMAE. 2
Potential neurotoxicity
Monomethyl Auristatin E, a potent cytotoxic agent used in Antibody-Drug Conjugates for cancer treatment, has raised concerns regarding its potential neurotoxicity. Clinical observations increasingly report cases of peripheral neuropathy associated with Monomethyl Auristatin E-containing ADCs. Peripheral neuropathy encompasses a range of disorders affecting the peripheral nervous system, characterized by symptoms such as weakness, numbness, and pain. This condition can significantly impact the course of treatment and the quality of life of patients, leading to prolonged infusion times or necessitating dose reductions. In severe cases, peripheral neuropathy may even pose a life-threatening risk. The higher incidence of peripheral neuropathy with Monomethyl Auristatin E-linked ADCs highlights the importance of closely monitoring patients for neurological symptoms and adjusting therapy accordingly to mitigate these adverse effects. Understanding and managing Monomethyl Auristatin E's neurotoxic potential is crucial for optimizing the therapeutic efficacy and safety of ADCs incorporating this cytotoxic molecule. 3
Reference
1. Best RL, LaPointe NE, Azarenko O, et al. Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol Appl Pharmacol. 2021;421:115534.
2. Zhou W, Fang P, Yu D, et al. Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E-based Antibody-Drug Conjugate for Treatment of Nectin-4-expressing Cancers. Mol Cancer Ther. 2023;22(8):913-925.
3. Fu Z, Gao C, Wu T, et al. Peripheral neuropathy associated with monomethyl auristatin E-based antibody-drug conjugates. iScience. 2023;26(10):107778.
You may like
Related articles And Qustion
Lastest Price from MonoMethyl auristatin E manufacturers

US $30.00/kg2023-09-07
- CAS:
- 474645-27-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000t/year
![474645-27-7 MMAE;[3H]-Vedotin](/ProductImageEN/2021-07/Small/fa121420-61aa-42c9-9636-39edf05c31a7.jpg)
US $15.00-10.00/KG2021-07-13
- CAS:
- 474645-27-7
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton