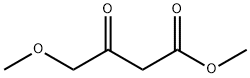
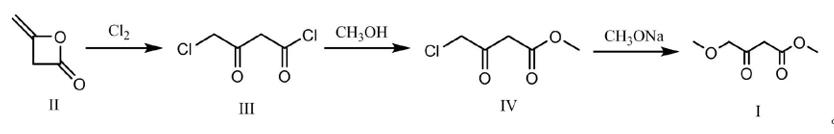
Methyl 4-methoxyacetoacetate - application on synthetic works
Methyl 4-methoxyacetoacetate has been used in generation of a small focused library of diversely functionalized dihydropyrimidine derivatives via one-pot three-component Biginelli cyclocondensation of β-ketoesters, aldehydes and (thio)ureas. Methyl 4-Methoxyacetoacetate is also a reactant in the formation of an abiotic poorphyrinogen in aqueous solution.
Application on synthetic works
The following example is about its application on the synthesis of N-hydroxyamidinoheterocycles [1]
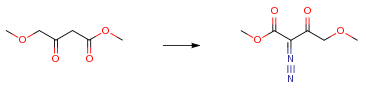
Methyl 4-methoxy-3-oxobutanoate (4.28 g, 0.029 mol) was dissolved in ether (20 mL). The solution was cooled in an ice bath. To the solution was added p-toluenesulfonyl azide (5.78 g, 0.029 mol) followed by N-ethylethanamine (2.0 mL, 0.019 mol). The solution was stirred at 0° C. for 15 minutes, then at rt for 30 minutes.
Upon evaporation, the tosyl amide bi-product solidified. This was filtered off and the filtrate was purified by flash chromatography to give the desired product (4.5 g, 89 percent) as a light oil.
The following example is about its application on the synthesis of pyridazinone compounds [2]
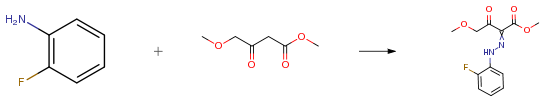
A solution of NaNO2 (1.66 g, 24 mmol) in H2O (5 mL) was added dropwise at 0° C. to a mixture of 2-fluoroaniline (1.93 mL, 20 mmol) and 6 M HCl aqueous solution (20 mL, 120 mmol). After stirring for 15 min, the resulting aqueous solution was added to a suspension of methyl 4-methoxyacetoacetate (2.59 mL, 20 mmol) and NaOAc (9.84 g, 120 mmol) in MeOH (40 mL) pre-cooled at 0° C. The reaction mixture was poured into water and extracted with AcOEt. The extract was washed with saturated NaHCO3 aqueous solution and brine, dried over MgSO4, and concentrated under reduced pressure. The residue was recrystallized from hexane/AcOEt to give the title compound (5.03 g, 94percent yield) as pale yellow crystals
The following example is about its application on the synthesis of pyrimidinyl indole compounds [3]
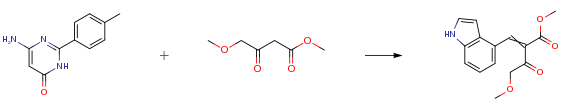
To a solution of 4-methoxy-3-oxo-butyric acid methylester (1 g, 6.88 mmol) in anhydrous isopropanol (10 mL) were added lH-indole-4-carbaldehyde (0.3 g, 4.39 mmol) followed by catalytic amount of piperidine acetate (0.1 g, 0.68 mmol) and the reaction mixture was stirred at ambient temperature for 6 hours. The separated yellow solid was filtered to get 2-(lH-indol-4-ylmethylene)-4-methoxy-3-oxo-butyric acid methyl ester in 75 percent yield (1.41 g).
The following example is about its application on the synthesis of novel 2-oxindole scaffold as a highly potent and brain-penetrant phosphodiesterase 10A inhibitor [4]
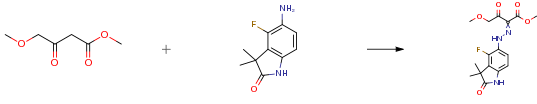
A solution of sodium nitrite (0.081 g, 1.17 mmol) in water (1 mL) was added dropwise to a solution of staring material (0.19 g, 0.978 mmol) in 6 M HCl aq (2 mL) at 0°C. After stirring at 0°C for 15 min, the mixture was added to a suspension of methyl 4-methoxyacetoacetate (0.127 mL, 0.978 mmol) and sodium acetate (1.0 g, 12.19 mmol) in MeOH (5 mL) at 0°C. The formed precipitate was dissolved in EtOAc, and the solution was washed with water and saturated NaHCO3 aqueous solution. The organic layer was dried over MgSO4, and concentrated under reduced pressure to give the product (0.25 g, 73 percent) as a yellow solid.
References
1.Combs AP,Takvorian A, Zhu W. Sparks R. N-hydroxyamidinoheterocycles as modulators of indoleamine 2,3-dioxygenase. US2007/185165[P], 2007, A1, Page column 57.
2.Taniguchi T, Kawada A, Kondo M, Quinn JF, Kunitomo J, Yoshikawa MF. Pyridazinone compounds. MakotoUS2010/197651[P], 2010, A1, Page column 60.
3.CHEMOCENTRYX. Substituted dihydropyridines and methods of use, NC.WO2007/51062[P], 2007, A2, Page column 155-156.
4.Yoshikawa M, Kamisaki H, Kunitomo J, Oki H, Kokubo H, Suzuki A, Ikemoto T, Kimura H, Taniguchi T. Design and synthesis of a novel 2-oxindole scaffold as a highly potent and brain-penetrant phosphodiesterase 10A inhibitor. Bioorganic and Medicinal Chemistry, 2015, 23(22):7138-7149.
5.https://iaspub.epa.gov/sor_internet/registry/substreg/searchandretrieve/advancedsearch/externalSearch.do?p_type=CASNO&p_value=41051-15-4
6.https://pubchem.ncbi.nlm.nih.gov/compound/123500
Related articles And Qustion
See also
Lastest Price from Methyl 4-methoxyacetoacetate manufacturers

US $0.00/KG2025-04-15
- CAS:
- 41051-15-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/KG2025-04-04
- CAS:
- 41051-15-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton