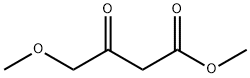
Methyl 4-methoxyacetoacetate:a synthetic intermediate
Introduction
Methyl 4-methoxyacetoacetate is often used as a synthetic intermediate in organic chemistry. It serves as a building block in the synthesis of more complex organic molecules. Compounds with acetoacetate moieties, like this one, are valuable in medicinal chemistry and other fields where specific functional groups are required. Colorless transparent liquid with a fragrant aroma. Miscible with ethanol and ether, slightly soluble in water.
Application
4-Methoxyacetoacetate methyl ester is commonly used as a synthetic intermediate in organic chemistry. It serves as a building block when synthesizing more complex organic molecules. Compounds with acetyl acetate groups are of great value in pharmaceutical chemistry and other fields that require specific functional groups. An important organic synthesis intermediate widely used in chemical, pharmaceutical and other industries. In drug synthesis, this product is mainly used to synthesize calcium ion antagonists for treating hypertension. With the continuous development of technology, the dosage and use of methyl dimethoxyacetoacetate will continue to expand. At present, there are no relevant research reports in China, so it is necessary to study and innovate the synthesis process of this intermediate1. Methyl 4-methoxyacetoacetate is an important pharmaceutical intermediate. At present, this compound is mainly used for the synthesis of new anti AIDS drugs Dolutegravir, Bictegravir and Cabotegravir. Dolutegravir is an anti AIDS integrase inhibitor approved by the US FDA in 2013. 4-Methoxyacetoacetate methyl ester is a key starting material for the synthesis of three drugs, and its quality and price have a significant impact on the synthesis of drugs. Methyl 4-methoxyacetoacetate was an important intermediate of dolutegravir. Dolutegravir, approved by the U.S. FDA in 2013, is an integrase strand transfer inhibitor used in the treatment of HIV/AIDS. Compared to existing HIV integrase inhibitors such as raltegravir and elvitegravir, dolutegravir exhibits improved safety. In contrast to Merck's antiretroviral drug raltegravir, not only did dolutegravir achieve comparable efficacy in Phase III clinical trials, but it also does not require co-administration with pharmacokinetic enhancers. Furthermore, dolutegravir demonstrates potent resistance properties and only needs to be taken once a day2.
Synthesis
Methyl 4-methoxyacetoacetate was prepared in a total yield of 93% from 4-chloroacetoacetic acid methyl ester via substitution reaction with methanol in the presence of NaH and CH3 OK to give organic salts,followed by acidification and decoloration. A method for the preparation of methyl 4-methoxyacetoacetate is described, comprising the following sequential steps: (a) Compound II is dissolved in dichloromethane, and the resulting solution is introduced into a microchannel reactor. Chlorine gas is then introduced to facilitate chlorination, yielding Compound III. (b) Methanol is pumped into the microchannel reactor to conduct an esterification reaction, resulting in a reaction mixture containing Compound IV. (c) The reaction mixture containing Compound IV undergoes a water wash to remove dichloromethane, yielding crude Compound IV. (d) Acetonitrile is introduced into a reaction vessel, nitrogen gas is purged, and solid sodium methoxide is added with stirring. The crude Compound IV is then dropwise added for methoxylation. (e) The reaction mixture is dropwise added to a 2% acetic acid solution while concurrently adding concentrated hydrochloric acid. The pH of the solution is maintained within the range of 4 to 8 during the addition process, ultimately controlling the pH to fall within the range of 6.0 to 7.0. (f) After allowing the layers to settle, the aqueous phase is extracted using acetonitrile. The organic phase is combined and subjected to a rotary evaporation process to remove acetonitrile, maintaining a temperature of 50°C and a pressure of 100 Pa, yielding crude Compound I. (g) The crude Compound I is subjected to molecular distillation to obtain pure methyl 4-methoxyacetoacetat1.
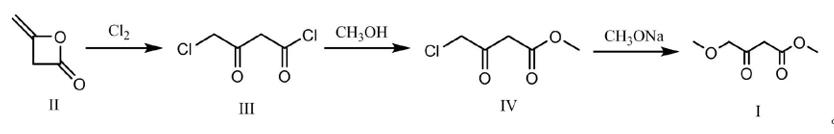
Figure 1 Synthesis of methyl 4-methoxyacetoacetate
Safety
Methyl 4-methoxyacetoacetate was an important intermediate of dolutegravir, may be associated with various side effects. Common side effects include nausea, headache, insomnia, fatigue, diarrhea, abnormal dreams, and, less commonly, skin rash. Additionally, some individuals may experience elevated liver enzymes. It's important to note that the severity of these side effects can vary among individuals, and not everyone will experience them. Serious but rare side effects, such as severe allergic reactions and changes in liver function, may also occur. Individuals taking dolutegravir should promptly report any side effects to their healthcare provider for guidance and potential adjustments to their treatment plan. Regular monitoring and communication with a healthcare professional are crucial for managing HIV treatment effectively3.
Reference
1. Guo Jianrong, Chang Honghong, Liu Qiang, et al. Study on the synthesis of dimethyl acetoacetate [J]. Shanxi Chemical Industry, 2003 (03): 17-19.
2. Blair HA. Dolutegravir/Rilpivirine: A Review in HIV-1 Infection. Drugs. 2018 Nov;78(16):1741-1750.
3. Scott LJ. Dolutegravir/Lamivudine Single-Tablet Regimen: A Review in HIV-1 Infection. Drugs. 2020 Jan;80(1):61-72.
You may like
Related articles And Qustion
Lastest Price from Methyl 4-methoxyacetoacetate manufacturers

US $0.00/KG2025-04-15
- CAS:
- 41051-15-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/KG2025-04-04
- CAS:
- 41051-15-4
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton