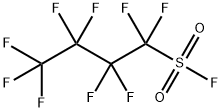
Method for producing Perfluorobutanesulfonyl fluoride
Nonafluorobutanesulfonyl fluoride also called Perfluorobutanesulfonyl fluoride is a versatile compound in organic synthesis. It can be used as a fluoride source for the nucleophilic introduction of fluorine, but it is also frequently applied as sulfonylation reagent generating intermediates with strong electron withdrawing perfluorinated alkyl substituents.
The method for producing perfluorobutanesulfonyl fluoride according to this embodiment is characterized in that an aqueous solution of an alkali metal hydroxide is added to perfluorobutanesulfonyl fluoride for purification. According to this production method (purification method), the content of perfluorosulfolane in perfluorobutanesulfonyl fluoride can be reduced to 100 ppm or less in 1 to 2 hours. Hereinafter, the reaction that occurs when an aqueous solution of an alkali metal hydroxide is added to perfluorobutanesulfonyl fluoride containing perfluorosulfolane as an impurity will be described in the case of using potassium as the alkali metal.
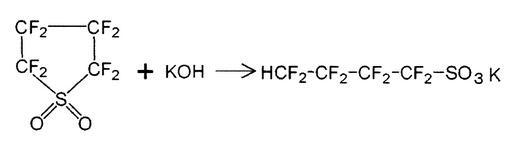
Both perfluorobutanesulfonyl fluoride and perfluorosulfolane are insoluble in water. Here, when an aqueous solution of potassium hydroxide is added, at a reaction temperature of 0 to 50 ° C. (preferably 20 to 30 ° C.), perfluorobutanesulfonyl fluoride does not react with potassium hydroxide and does not change. On the other hand, perfluorosulfolan reacts with potassium hydroxide to produce water-soluble 1,1,2,2,3,3,4,4-octafluorobutanesulfonic acid potassium salt by the above reaction formula. Dissolve in an aqueous solution of potassium hydroxide. After the reaction of the reaction formula is completed and 1,1,2,2,3,3,4,4-octafluorobutanesulfonic acid potassium salt is completely dissolved in an aqueous solution of potassium hydroxide, The aqueous solution of potassium hydroxide containing perfluorobutanesulfonyl fluoride and 1,1,2,2,3,3,4,4-octafluorobutanesulfonic acid potassium salt is separated into layers. Perfluorobutanesulfonyl fluoride is separated into a lower layer, and an aqueous solution of potassium hydroxide is separated into an upper layer. Therefore, high-purity perfluorobutanesulfonyl fluoride can be obtained by separating only the lower layer.
The alkali metal hydroxide is preferably at least one selected from the group consisting of lithium hydroxide, sodium hydroxide, potassium hydroxide, rubidium hydroxide, and cesium hydroxide.
Alkaline metal hydroxide aqueous solution: The amount of the alkali metal hydroxide is preferably 0.1 to 20 parts by mass, more preferably 1 to 5 parts by mass with respect to 100 parts by mass. When the ratio of the alkali metal hydroxide is less than 0.1 parts by mass, no purification effect is observed. When the ratio of the alkali metal hydroxide exceeds 20 parts by mass, decomposition of perfluorobutanesulfonyl fluoride occurs, which is not preferable.
In the removal of perfluorosulfolane, the addition amount (ratio) of alkali metal hydroxide is preferably 1 to 10 mol, more preferably 2 to 5 mol, per mol of perfluorosulfolane. When the ratio of the alkali metal hydroxide is less than 1 mol, the reaction of the reaction formula (3) is not completed and the purification effect cannot be obtained. Even if the ratio of the alkali metal hydroxide exceeds 10 mol, a remarkable effect cannot be obtained.
The addition of an aqueous solution of an alkali metal hydroxide to perfluorobutanesulfonyl fluoride may be performed by a known method and is not particularly limited. However, in order to easily take out the lower layer perfluorobutanesulfonyl fluoride separated after the purification, it is preferable to use, for example, a separatory funnel. An example of the reaction conditions is a reaction for 1 to 2 hours at room temperature while appropriately stirring the mixed solution. The reaction conditions may be appropriately determined depending on the amount of perfluorosulfolane contained in the perfluorobutanesulfonyl fluoride, the size of the batch to be reacted (amount of raw material), the strength of stirring, and the like.
Related articles And Qustion
See also
Lastest Price from Nonafluorobutanesulfonyl fluoride manufacturers
US $0.00/KG2025-11-21
- CAS:
- 375-72-4
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons

US $0.00/KG2025-04-21
- CAS:
- 375-72-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month