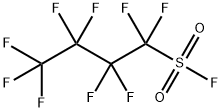
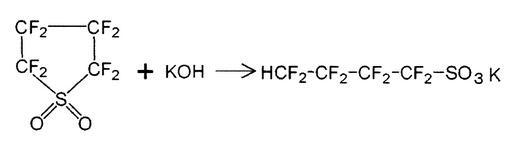
Benzyne domino process using Nonafluorobutanesulfonyl fluoride (NfF) as raw material
Benzynes were generated from o-(trimethylsilyl)phenols using nonafluorobutanesulfonyl fluoride (NfF) by a domino process, i.e., the nonaflation of the phenolic hydroxyl group of o-(trimethylsilyl)phenols by NfF followed by the attack of the produced fluoride ion on the trimethylsilyl group. The generated benzyne immediately underwent various reactions to give polysubstituted benzenes.
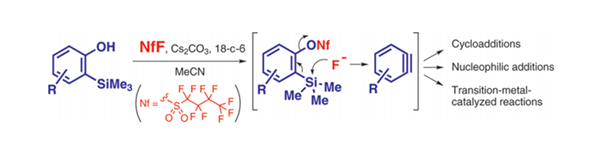
we have developed a domino process for benzyne preparation from o-(trimethylsilyl)phenols 3 using NfF, which is one of the most stable and least expensive perfluoroalkanesulfonylating reagents. The single reagent, NfF, promotes benzyne generation through two successive steps: the activation of the phenolic hydroxyl group of 3 by the formation of the nonaflate and the elimination of the trimethylsilyl group of 3 via nucleophilic attack by its fluoride moiety. Acid and/or fluoride ion labile functional groups, such as a cyclic acetal and an O-TBDMS group, were compatible under the developed conditions.
References:
[1] TAKASHI IKAWA. A Domino Process for Benzyne Preparation: Dual Activation of o-(Trimethylsilyl)phenols by Nonafluorobutanesulfonyl Fluoride[J]. Organic Letters, 2011, 13 7: 1730-1733. DOI:10.1021/ol200252c.
Related articles And Qustion
Lastest Price from Nonafluorobutanesulfonyl fluoride manufacturers
US $0.00/KG2025-11-21
- CAS:
- 375-72-4
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons

US $0.00/KG2025-04-21
- CAS:
- 375-72-4
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month