Mechanism and Pharmacodynamics of Rilopirox
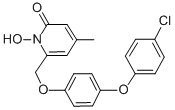
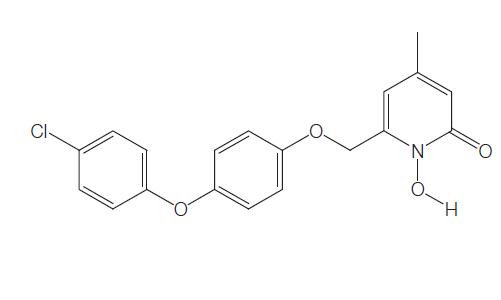
Rilopirox, 6-[[p-(p-chlorophenoxy)phenoxy]methyl]-1-hydroxy-4- methyl-2(1H)-pyridone, is a new water insoluble fungicidal antimycotic. It is the second antifungal agent of the hydroxypyridone antifungals, along with ciclopirox. In contrast with ciclopirox olamine, rilopirox exhibits strong activity against yeast, especially Candida albicans.
Rilopirox was first developed as an intravaginal antifungal. However, recent studies suggest a role for this drug in the treatment of tinea versicolor, seborrheic dermatitis, and oropharyngeal candidiasis. The chemical structure of rilopirox is shown below.
Uses
Rilopirox is potentially likely to be useful in the treatment of vaginal candidiasis, seborrheic dermatitis, and tinea versicolor. However, there are currently no clinical studies available to confirm these indications.
Mechanism of action
The antifungal mode of action of rilopirox is similar to that of ciclopirox. Rilopirox acts by damaging the cell membrane of the fungus and impairing several metabolic enzyme systems through its strong chelating action, thus inhibiting iron-dependent enzymes. Rilopirox inhibits the iron-containing enzyme, catalase.
Yeast catalase splits hydrogen peroxide into water and oxygen. Rilopirox acts by inhibiting catalase activity, leading to accumulation of the toxic compound, hydrogen peroxide. This causes irreversible fungal cell damage. Another metabolic iron-dependent enzyme of the yeast mitochondria respiratory chain, the complex 1(NADH-ubiquinone oxidoreductase), is also inhibited by the chelating effects of rilopirox. Rilopirox shows a high killing rate, even against nonproliferating fungi and spores. Its unique mode of action explains its fungicidal activity against yeasts.
Medication mode and dosage
Rilopirox is available as topical preparation for the treatment of vaginal candidiasis, seborrheic dermatitis, and tinea versicolor. However, no clinical indications and dose recommendations are currently available.
Pharmacokinetics and Pharmacodynamics
Rilopirox is a hydrophobic topical agent. In vitro studies by Harada et al. demonstrated that the activity of rilopirox was not affected by changes in pH or adding serum to culture media. These qualities have made rilopirox a candidate in the treatment of oral and vulvovaginal candidal infections. Preliminary pharmacokinetic studies in dogs treated with 1 mg/kg body weight of 14C-labeled rilopirox intravaginally have demonstrated a maximum serum concentration of 28 mg/ml. Oral treatment with 20 mg/kg body weight led to a maximum serum concentration of 478 mg/l. These measurements were performed 1–3 hours post dose.
After 24 hours, serum drug concentrations remained below the detection level of 10 mg/l. In a study of healthy female volunteers, individuals were treated intravaginally with rilopirox 22.5 mg daily for 5 consecutive days. With the exception of one subject, serum rilopirox concentrations remained within the threshold level between 10 and 20 mg/l in all participants.