Mechanism and Side-effects of Flucytosine
Flucytosine (Ancotil, Ancobon) is a fluorinated pyrimidine analog that is primarily used in combination with amphotericin B or fluconazole for the treatment of invasive yeast infections. Rapid emergence of drug resistance is observed when this agent is used as monotherapy.
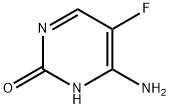
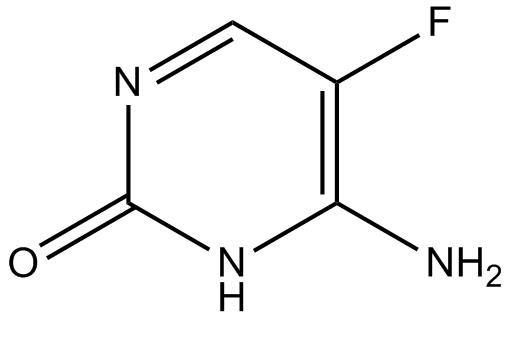
Flucytosine is a small polar molecule with a molecular weight of 120
daltons and a log P value of –0.89; the chemical structure is shown below. This compound was developed in 1957 at Roche
Laboratories as an antineoplastic agent. Flucytosine does not have
cytotoxic activity, but was demonstrated to have antifungal activity.
Flucytosine is most commonly used for the treatment of cryptococcal
meningitis and severe Candida infections that are refractory to firstline
antifungal agents or those occurring within sanctuary sites.
Flucytosine is available as tablets (500 and 250 mg flucytosine) and as
an intravenous formulation (2.5 g of flucytosine in 250 ml normal
saline).
Mechanism of action
Flucytosine is metabolized via the pyrimidine salvage pathway, where it acts as a subversive substrate. Metabolism produces toxic nucleotides that interfere with nucleic acid and protein synthesis. Flucytosine is transported into fungi by membrane permeases, where it is deaminated by the protein cytosine deaminase to produce 5-fluorouracil (5-FU). 5-FU is then converted to 5-fluoro-uridylate (5-fluoro-UMP, 5-FUMP) by the protein uracil phosphoribosyltransferase (UPRT). 5-FUMP is sequentially phosphorylated to form 5-fluoro-UTP, which is incorporated into RNA. 5-FUMP is also reduced to 5-fluoro-2u-doexyuridylate, which inhibits the enzyme thymidylate synthetase. This leads to reduced DNA synthesis because of a reduction in the available nucleotide pool. The differential activity of flucytosine is due to the absence of cytosine deaminase in mammalian cells.
Bioavailability
The oral bioavailability of flucytosine is 90% in normal volunteers and patients in North America, but may be less in patients with HIV/AIDS. Co-administration with antacids may slow absorption, but probably has little effect on overall systemic exposure and clinical efficacy. Peak concentrations are observed 1–2 hours post oral dose. The mean half-life is 3–6 hours in patients with normal renal function, but may extend to several days in patients with renal failure. Protein binding is negligible. The administration of flucytosine 100 mg/kg orally results in a lower plasma peak concentrations, lower trough concentrations and lower AUCs than the equivalent dosage administered i.v in HIV/AIDS patients with cryptococcal meningitis, which may relate to suboptimal absorption. Oral administration results in higher concentrations of 5-FU than parenteral therapy, presumably because of conversion of flucytosine by intestinal bacteria.
Side-effects
Gastrointestinal side-effects
Oral flucytosine is generally well tolerated, but occasionally causes nausea and diarrhea. More severe gastrointestinal symptoms, such as vomiting, abdominal pain, and profuse diarrhea, may occur. Ulcerating enterocolitis has been described with resolution following cessation of drug.
Hepatotoxicity
Transient hepatomegaly and elevated transaminase levels are occasionally seen and are more likely with sustained high peak serum levels (W100 mg/ml). Abnormal liver function is usually readily reversible. Extensive liver cell necrosis and fatal bone marrow depression have been reported in two patients, but this is extremely uncommon. Frequent monitoring of liver function tests is required to identify hepatotoxicity at the earliest possible time. Flucytosine can be safely used in patients with pre-existing liver disease.
Related articles And Qustion
See also
Lastest Price from 5-Fluorocytosine manufacturers
US $0.00/kg2025-11-21
- CAS:
- 2022-85-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/kg2025-09-01
- CAS:
- 2022-85-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons