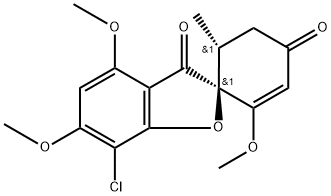
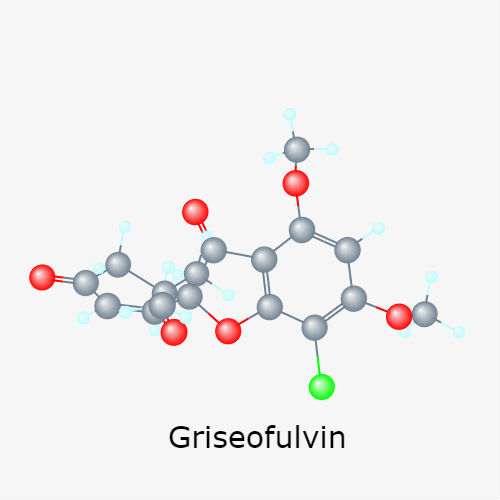
Mechanism of action of Griseofulvin
Griseofulvin (MW 352.5 daltons) is a metabolite of Penicillium griseofulvum and P. janczewskii that disrupts the fungal mitotic spindle and inhibits cell wall synthesis. The structure is shown below. This compound was discovered in 1939, but was not initially developed because of the absence of antibacterial activity.
Griseofulvin was developed as systemic therapy for Botrytis infection in lettuce and Alternaria blight of tomatoes, but was too expensive for widespread horticultural use. The clinical potential of this agent was not realized until 1958, when griseofulvin was demonstrated to be effective in laboratory animal models of Microsporum canis and Trichophyton mentagrophytes infection. Subsequently, griseofulvin was demonstrated to be effective for human dermatophyte infections. The advent of modern antifungal agents that exhibit more favorable pharmacokinetic and toxicity profiles has largely relegated griseofulvin to a second-line agent.
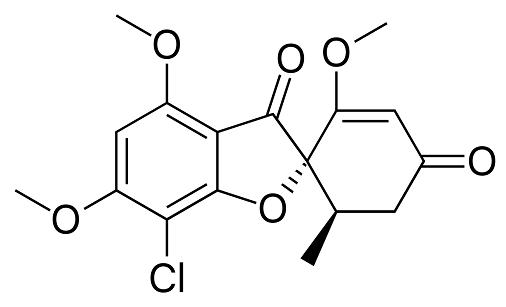
Mechanism of action
Griseofulvin inhibits the formation of intracellular microtubules and disrupts the mitotic spindle. Griseofulvin binds to tubulin and disrupts both the a- and b-subunits by inducing conformational changes. A decrease in transcript levels is seen. Because microtubules are involved in the transport of secretory material through the cytoplasm to the periphery of the cell, destruction of microtubules may lead to impaired processing of newly synthesized cell wall constituents at the growing tips of hyphae.
Bioavailability
Griseofulvin is poorly and erratically orally bioavailable. Absorption is improved with micronized formulations or by administration with a fatty or high-carbohydrate meal. The administration of griseofulvin with a fatty meal leads to approximately double the systemic exposure. Attempts to increase the bioavailability include micronization, solid solutions with polyethylene glycol and sodium dodecyl sulfate, emulsified formulations, freeze-dried emulsions, cyclodextrin formulations, and formation of nanoparticles from water-dilutable microemulsions. Peak concentrations are observed 2–4 hours post dose. The half life is 9.5–42 hours. Although oral bioavailability is improved with the ultramicrosize formulation, differences in systemic absorption between microsize and ultramicrosize formulations are minimized if the former is administered with a fatty meal. Protein binding is 85%.
Drug interactions
Griseofulvin reduces the anticoagulant effect of warfarin, presumably via induction of cytochrome P450 enzymes. A weak disufiram-like reaction can occur if griseofulvin is coadministered with alcohol. Griseofulvin may impair the performance of skilled tasks (e.g. driving) and enhances the effects of alcohol. Concomitant administration of barbiturates diminishes the absorption of griseofulvin. Griseofulvin administration leads to accelerated estrogen and progesterone metabolism that may impair the efficacy of the oral contraceptive and other hormonal therapies. Griseofulvin reduces serum salicylate concentrations and may reduce ciclosporin levels.
Side-effects
Gastrointestinal side-effects These are the most common side-effects and include nausea, vomiting, diarrhea, heartburn, flatulence, angular stomatitis, glossodynia, thirst, and a black-furred tongue.
Renal side-effects
Albuminuria and cylindruria without evidence of renal insufficiency have been described. There is a report of membranous glomerulopathy and nephrotic syndrome in a 16-year-old male who also appeared to develop evidence of systemic lupus erythematosus after treatment with griseofulvin. Interstitial nephritis manifest as hematuria, pyuria, and eosinophiluria with renal impairment associated with chronic griseofulvin therapy for onychomycosis has also been reported.
Myositis and a proximal myopathy secondary to griseofulvin treatment have been reported. Hepatotoxicity is infrequent, generally mild and usually reversible on cessation of the drug. Pre-existing liver disease may be exacerbated by griseofulvin; severe liver disease and hepatocellular failure are absolute contraindications to the use of griseofulvin.
Related articles And Qustion
See also
Lastest Price from (+)-Griseofulvin manufacturers

US $5.00-0.50/KG2025-05-07
- CAS:
- 126-07-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00-0.00/KG2025-04-21
- CAS:
- 126-07-8
- Min. Order:
- 1KG
- Purity:
- 98%min, BP2013/EP8
- Supply Ability:
- 5000kg/month