Liposomal bupivacaine: Overview, Efficacy in Postoperative Pain and Safety
General Description
Liposomal bupivacaine, marketed as DepoFoam bupivacaine (EXPAREL), is an extended-release formulation approved by the FDA in 2011. It offers prolonged postoperative pain control with a favorable safety profile. Studies show it is well tolerated with fewer serious side effects compared to traditional bupivacaine. Adverse reactions include nausea, vomiting, and headache. Research indicates potential myotoxicity and inflammation, necessitating monitoring for tissue injury. Metabolism primarily occurs in the liver, with minimal renal excretion. While efficacy has been demonstrated in specific surgeries, caution is advised for broader applications pending further validation. Compliance with guidelines is essential for safe use.

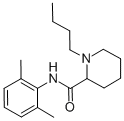
Figure 1. Bupivacaine
Overview
Liposomal bupivacaine, marketed as DepoFoam bupivacaine (EXPAREL), is an extended-release bupivacaine formulation approved by the FDA in October 2011. This formulation consists of bupivacaine encapsulated in lipid-based particles, forming multiple aqueous chambers resembling a spherical honeycomb. As the body resorbs the lipid walls, unaltered bupivacaine is slowly released into the surrounding tissue, allowing for drug diffusion over 96 hours after a single administration. Currently, it is available in a 20 mL single-use vial at a concentration of 1.3% (13.3 mg/mL), with the maximum recommended dosage being 266 mg. A pharmacokinetic study comparing subcutaneous injection of 2% liposomal bupivacaine and 0.5% plain bupivacaine found no difference in Cmax between the two groups. However, the liposomal bupivacaine group displayed a four-fold increase in bupivacaine dose and a 9.8-fold increase in terminal half-life, with a seven-fold increase in Tmax attributed to the slow release of the drug. These findings were confirmed in subsequent Phase II multicenter clinical trials, indicating the sustained and extended release properties of liposomal bupivacaine. 1
Efficacy in postoperative pain
Liposomal bupivacaine, currently indicated for localized pain relief after bunionectomy and hemorrhoidectomy, has demonstrated efficacy in postoperative pain management. However, its approval for other routes of administration such as intra-articular use, peripheral nerve blocks, and intravascular use is still pending, with ongoing trials investigating its potential applications [7]. In light of this, the Food and Drug Administration (FDA) issued a Warning Letter in September 2014 regarding the promotion of liposomal bupivacaine for surgeries beyond those for which it had been shown to be effective, leading to corrective messaging being ordered for all affected audiences. Additionally, based on Phase III clinical trials, the FDA mandated that Pacira issue corrective messaging emphasizing that "pain control beyond 24 h has not been demonstrated." Despite these regulatory actions, the demonstrated efficacy of liposomal bupivacaine in postoperative pain management for specific surgeries suggests promising potential for broader applications pending further clinical validation. 1
Safety
Liposomal bupivacaine, touted for its extended-release properties and improved postoperative pain control, has shown a promising safety profile in various studies. Compared to traditional bupivacaine HCL and control groups, liposomal bupivacaine has been well tolerated, with fewer incidences of serious side effects. Common adverse reactions reported in clinical trials include nausea, vomiting, constipation, pyrexia, dizziness, and headache. Studies have also delved into the potential myotoxicity, inflammation, and neurotoxicity associated with prolonged-duration local analgesia systems like EXPAREL. Research by McAlvin et al demonstrated that while EXPAREL exhibited longer sensory block duration compared to standard bupivacaine HCL, it caused similar myotoxicity as lower concentration bupivacaine HCL and increased inflammation, warranting continued monitoring for local tissue injury. Moreover, Richard et al's study on multidose administration of EXPAREL in animals highlighted its good tolerability, with lower peak plasma concentrations and minimal local tissue damage observed. However, caution is advised against coadministration with other local anesthetics or contact with certain antiseptics to prevent carrier disruption and potential toxicity risks. Notably, the metabolism of liposomal bupivacaine primarily occurs in the liver, with minimal unchanged excretion in urine. While studies suggest limited dose adjustments are needed in patients with moderate hepatic impairment, the safety of liposomal bupivacaine remains unestablished for pediatric and obstetric populations. Vigilance and adherence to pharmaceutical guidelines are crucial to ensuring the safe and effective use of DepoFoam bupivacaine. 2
Reference
1. Uskova A, O'Connor JE. Liposomal bupivacaine for regional anesthesia. Curr Opin Anaesthesiol. 2015;28(5):593-597.
2. Zhang X, Yang Q, Zhang Z. The efficiency and safety of local liposomal bupivacaine infiltration for pain control in total hip arthroplasty: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(49):e8433.
Related articles And Qustion
See also
Lastest Price from Bupivacaine manufacturers

US $0.00/kg2025-04-22
- CAS:
- 2180-92-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 2180-92-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 800kg