The synthesis routes and applications of atropic acid
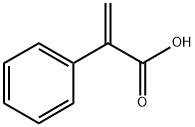
Atropic acid is an unsaturated carboxylic acid containing a benzene ring and an acryloyl group. It is widely found in nature, such as in the essential oils of cinnamon bark, osmanthus and other plants.
Synthesis
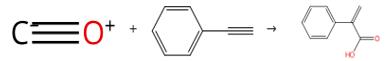
Fig. 1 The synthesis route of atropic acid
Carry out the carbonylation reactions in 50 mL stainless steel high-pressure reactors fitted with PTFE inserts. Heat the reactors using oil baths. Stir the reaction mixture with the aid of magnetic stirrer bars. Dissolve the calculated amounts of Pd catalyst, ligand, co-promoter, and substrate in the solvent of interest as detailed in the tables. Introduce the solution to the reactor vessel. Flush the reactor three times with carbon monoxide. Seal the reactor vessel. Pressurize the reactor vessel to 35 bar or as otherwise indicated. Heat the mixture to the required temperature for the specified period of time. After cooling to room temperature, depressurize the reactor. Distill the product from the catalyst under vacuum (150 °C, 0.01 mm Hg). Analyze the product using 1H and 13C NMR spectroscopy and GC-fID analysis. After completion of the reaction, remove the methanol solvent under vacuum. Add DCM (30 mL) to the residue. Wash the solution with water (3 x 10 mL). Dry the organic phase over magnesium sulfate. Remove the solvent under vacuum. Dissolve the residue in methanol. The synthesis route is shown in Fig.2[1].
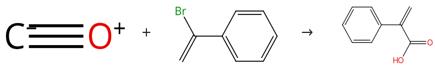
Fig. 2 The synthesis route of atropic acid
Add Pd(PPh3)2Cl2 (5 mol %), [BMIM]-PF6 (2.0 g), β-bromostyrene (2 mmol), water (0.18 mL, 10 mmol), and Et3N (0.57 mL, 4 mmol) into a 45-mL autoclave containing a glass liner and a magnetic stirring bar. Flush the autoclave three times with CO. Pressurize the autoclave to 20 bar of CO. Heat the mixture at 100 °C for 10 hours. Extract the resulting solution with water (3 mL x 4). Acidify the aqueous phase with aqueous hydrochloric acid (0.25 M). Extract the aqueous phase with diethyl ether (20 mL x 3). Combine the ether phases. Dry the ether phases over anhydrous MgSO4. Filter the ether phases through Celite. Concentrate the filtrate under reduced pressure. Wash the ionic liquid phase with 3 mL of diethyl ether. Add fresh starting material to the mixture to perform a second run reaction under the same conditions. Allow the reaction time for runs 2-5 to be 10, 10, 16 and 24 hours. After three runs, add 0.5 g of fresh ionic liquid to the mixture. The synthesis route is shown in Fig.2.
Applications
The unique structure of atropic acid allows it to be used as a starting material or key intermediate for the synthesis of a variety of complex organic compounds, such as drugs, fragrances, dyes, etc.
References
[1]Han, Xiaoyu; et al. Enantioselective Cycloaddition of Allenes to Acrylates Catalyzed by Dipeptide-Derived Phosphines: Facile Creation of Functionalized Cyclopentenes Containing Quaternary Stereogenic Centers. Journal of the American Chemical Society (2011), 133(6), 1726-1729.
Related articles And Qustion
See also
Lastest Price from Atropic acid manufacturers
US $1.00-100.00/KG2024-07-16
- CAS:
- 492-38-6
- Min. Order:
- 1KG
- Purity:
- 95%
- Supply Ability:
- 200000KG

US $9.00/KG2024-05-29
- CAS:
- 492-38-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000tons