Lactose: A Comprehensive Overview
Introduction
Lactose, a disaccharide sugar derived primarily from milk, has played a pivotal role in the fields of food science and pharmaceuticals. Composed of glucose and galactose, this biologically significant molecule presents a wealth of applications that extend beyond basic nutrition. The following article aims to elucidate the synthetic processes, core components, versatile applications, and optimal storage conditions of lactose, offering insights valuable to professionals in the chemical sector[1].

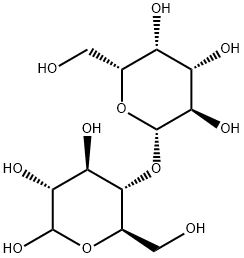
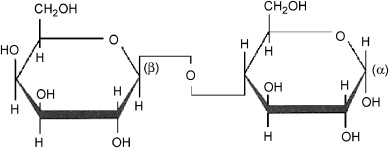
Figure 1 Characteristics of Lactose
Synthesis
The synthesis of lactose occurs naturally within the mammary glands of lactating mammals. This process involves the enzymatic reaction where glucose and UDP-galactose (uridine diphosphate galactose) converge, facilitated by the enzyme lactose synthase. In industrial settings, lactose is extracted from whey, a by-product of cheese production. The process typically includes concentration, crystallization, and drying steps to yield high-purity lactose suitable for various applications.
Main Components
Chemically, lactose is classified as a reducing sugar with the formula C12H22O11. It uniquely features a β-1,4 glycosidic bond linking the glucose and galactose molecules. This structural characteristic is critical, as it influences the sugar's reactivity and its interaction with other molecules, particularly during enzymatic browning and Maillard reactions which are pivotal in food science. The reducing nature of lactose allows it to partake in reduction-oxidation reactions that are essential in many biological and chemical processes.
In its pure form, lactose exists as a white, crystalline substance with a mildly sweet flavor and is slightly soluble in water. Its solubility increases with temperature, making it adaptable for various applications. The crystalline form of lactose can be further categorized into two polymorphs: alpha-lactose and beta-lactose. Alpha-lactose, the more stable form, predominates in lower temperatures and has a less sweet taste compared to beta-lactose, which is predominant at higher temperatures. This polymorphism affects not only the physical properties like solubility and sweetness but also the functional properties in pharmaceutical and food applications.
Applications
Lactose's utility spans several industries, primarily due to its functional properties as a filler, stabilizer, and sweetener. In the pharmaceutical sector, it is extensively used as an excipient in tablet formulation, helping to improve the compressibility and dissolution of active ingredients. This characteristic ensures that tablets not only maintain their shape during handling but also release their medicinal components effectively upon ingestion.
In food technology, lactose is pivotal, particularly in dairy products and as a standard in milk powders, enhancing texture and flavor. Its role extends to the production of caramel color in baked goods and beverages, where its controlled decomposition under heat contributes to desirable color and taste.
In more specialized applications, lactose serves as a substrate in microbiological media, aiding in the differentiation of lactose-fermenting and non-fermenting bacteria. This capability is crucial in both clinical diagnostics and food safety testing, where rapid identification of pathogens can prevent disease outbreaks. Additionally, lactose is employed in the production of bioethanol and bioplastics, marking its growing significance in sustainable technologies. These applications harness lactose's fermentable nature to produce environmentally friendly alternatives to conventional fuels and plastics, reflecting an innovative use of this natural sugar in addressing global sustainability challenges.
Storage
Proper storage of lactose is essential to maintain its quality and functional properties. Lactose should be stored in a cool, dry environment, away from sources of moisture and heat which can lead to clumping and degradation of the sugar. Packaging in air-tight containers is recommended to prevent the ingress of moisture and other contaminants. Under optimal conditions, lactose can maintain its quality for several years, although it is advisable to adhere to specific storage guidelines provided by manufacturers.
Conclusion
Lactose, with its multifaceted applications and significant role in various industries, remains a substance of great interest within the chemical and pharmaceutical landscapes. Understanding its synthesis, structure, and uses, along with proper storage techniques, can significantly enhance its utility in diverse fields. As research continues and technology advances, the potential for new and innovative applications of lactose is expansive, promising further enhancements in food science, medicine, and environmental sustainability[2].
![Article illustration]() References
References
[1]Swagerty Jr D L, Walling A D, Klein R M. Lactose intolerance[J]. American Family Physician, 2002, 65(9): 1845-1851.
[2]Vesa T H, Marteau P, Korpela R. Lactose intolerance[J]. Journal of the American College of Nutrition, 2000, 19(sup2): 165S-175S.
You may like
Related articles And Qustion
See also
Lastest Price from Lactose manufacturers

US $10.00/ASSAYS2025-08-19
- CAS:
- 63-42-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/KG2025-04-21
- CAS:
- 63-42-3
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month