Isoamyl Acetate: Physical Properties, Industrial Applications and Enzymatic Synthesis
Description
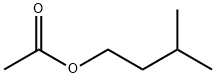
Isoamyl acetate is a colorless liquid with a fruity aroma, with the molecular formula C7H14O2. Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil or pear oil.

Physical Properties
Isoamyl acetate is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. It has a strong odor which is described as similar to both banana and pear.
Industrial Applications
Isoamyl acetate is used to confer banana or pear flavor in foods such as circus peanuts, Juicy Fruit and pear drops.It is also used as a solvent for some varnishes, oil paints, and nitrocellulose lacquers. As a solvent and carrier for materials such as nitrocellulose, it was extensively used in the aircraft industry for stiffening and wind-proofing fabric flying surfaces, where it and its derivatives were generally known as 'aircraft dope'. Now that most aircraft wings are made of metal, such use is mostly limited to historically accurate reproductions and scale models.
Because of its intense, pleasant odor and its low toxicity, isoamyl acetate is used to test the effectiveness of respirators or gas masks.
Enzymatic Synthesis
Isoamyl acetate was successfully synthesized from isoamyl alcohol in supercritical carbon dioxide by enzymatic catalysis. First, the impact of the acyl donor was investigated. Among several reactants, including acetic acid and two different acetates, acetic anhydride gave best yields. Then, two different immobilized lipases (Novozym 435 from Candida antarctica and Lipozyme RM-IM from Rhizomucor miehei) as biocatalysts for the above-mentioned reaction were compared. An esterification extent of 100% was obtained in continuous operation using acetic anhydride as acyl donor and Novozym 435 as enzyme. The amount of enzyme preparation was optimised to 6.25 g/mol alcohol. The effect of substrates load in the solvent was investigated. Operating at a CO2/substrates molar ratio below 7.0, the conversion of alcohol decreased, probably due to an inhibitory effect on enzyme by high concentration of acetic anydride or by produced acetic acid. Pressure in the range of 8–30 MPa showed no effect on this reaction, while an increase in temperature (over 313 K) led to lower production of isoamyl acetate. Novozym 435 was very stable not finding any loss of activity during one month of continuous operation.1
References:
[1] M.D. ROMERO . Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in supercritical carbon dioxide[J]. Journal of Supercritical Fluids, 2005, 33 1: 1-98. DOI:10.1016/j.supflu.2004.05.004.You may like
Lastest Price from Isoamyl acetate manufacturers

US $142.00/KG2025-03-26
- CAS:
- 123-92-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1-20mt
US $1.00/KG2025-03-14
- CAS:
- 123-92-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt