Is Ozone a polar molecule?
Physical and chemical properties
Ozone is an allotrope of oxygen much less stable than diatomic molecules. Ground-level Ozone is an air pollutant with harmful effects on the respiratory system, while the ozone layer in the upper atmosphere filters potentially damaging ultraviolet light from reaching the Earth's surface, which saves us from harmful ultraviolet rays coming from the Sun.
Ozone is a triatomic oxygen compound and its high reactivity results from its molecular structure. O3 is the chemical formula for Ozone. Chemically, it is a trioxygen gas molecule with a 48 g/mol molar mass. At standard conditions, Ozone is a pale blue gas; at concentration levels in the Earth's atmosphere, it is colorless. The ozone density is over 1.5 times greater than that of the air. Gaseous ozone mixtures with oxygen, nitrogen, or air are close to ideal mixtures. At concentrations of over 10-11% by volume or greater, ozone-air mixtures are explosive, as Ozone decomposes with the emission of large quantities of energy[1].
polar or non-polar
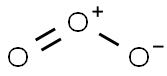
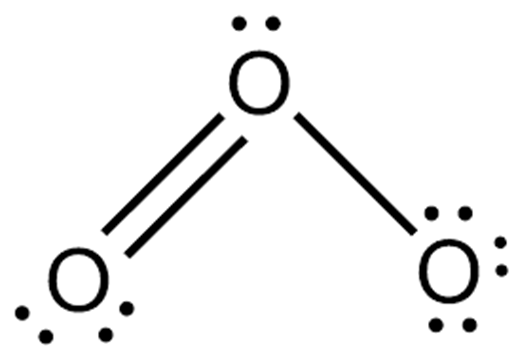
There is a very famous question regarding whether O3 is polar or non-polar. O3 consists of three identical oxygen (O) atoms. One oxygen atom is present at the center of the molecule, while the other two oxygen (O) atoms occupy terminal positions, one on each side. The central O-atom has a lone pair of electrons. Each oxygen atom in O3 has the same electronegativity value, i.e., 3.44 units. Therefore, no electronegativity difference exists between the bonded atoms in the O=O and O-O bonds present in the O3 molecule.
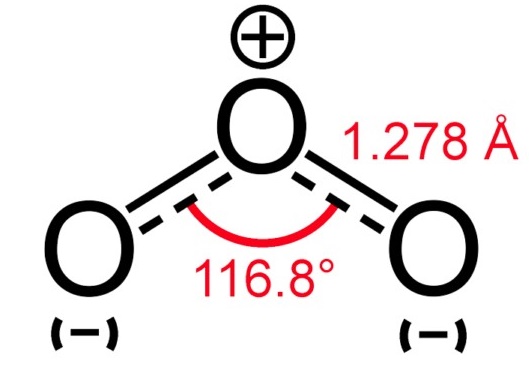
However, it is very well known that lone pair-lone pair repulsion is greater than bond pair-bond pair and lone pair-bond pair repulsion. Therefore, Oxygen atoms in Ozone face lone pair-lone pair repulsion that leads to a bent shape. O3 occupies a bent, angular, or V-shape.
The central O-atom carries a +1 formal charge, while the single-bonded O-atom carries a -1 formal charge in O3. The O-atom carrying +1 charge is slightly electron deficient, while the O-atom carrying -1 formal charge is slightly electron rich. The electron-deficient O-atom attracts O-O bonded electrons to a greater extent. Consequently, it is due to these formal charges and bent shape that O3 contains a non-uniform charge distribution overall. It is thus slightly polar (net µ = 0.53 D).
Use
Ozone automatically and rapidly decomposes to oxygen in both air and water, with an oxidation potential of 2.07 V. This high oxidative power and rapid decomposition make it effective against a broad spectrum of microorganisms; hence, its wide application for decontamination of indoor spaces, materials/surfaces, food, and water. Ozone yields faster microbial inactivation kinetics than other oxidative agents used in chemical disinfectants and is effective on notably resistant microorganisms such as Clostridium difficile [2]. It can react up to 3000 times faster with organic matter and is considered safer than chlorine, which produces harmful disinfection by-products. Beyond decontamination, Ozone also possesses bleaching and deodorizing properties, increasing its versatility in various industries.
References
[1] Greene, A. Z. Guzel‐Seydim and A. C. Seydim. “Chemical and Physical Properties of Ozone.” 2012. 0.
[2] Emmanuel I. Epelle . “Ozone application in different industries: A review of recent developments.” Chemical Engineering Journal 454 (2023): Article 140188.