How the Ozone lewis structure is formed
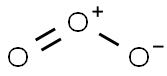
lewis structure of O3
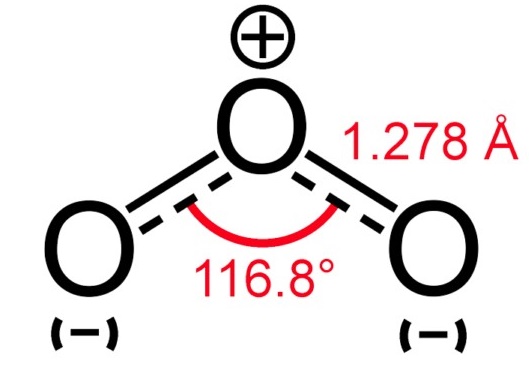
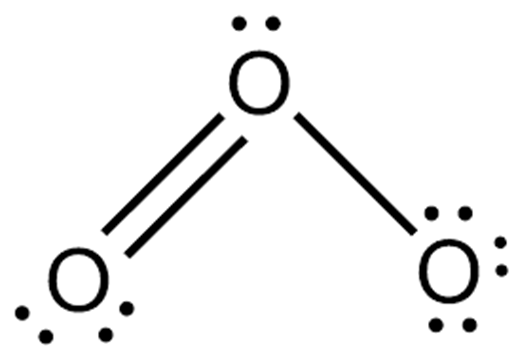
Ozone (O3) consists of three oxygen atoms (O) as oxygen isomers. O3 hybridises as a SP2 hybrid. The Lewis structure of O3 also consists of three oxygen atoms bonded at an angle of 116 degrees. There is one double bond and one single bond between the oxygen atoms (O). The central oxygen atom has one lone pair of electrons, the double bonded oxygen atom has two lone pairs of electrons, and the single bonded oxygen atom has three lone pairs of electrons. The structure is shown below:
Steps for drawing the O3 Lewis structure
Step 1 Calculate the number of valence electrons for O
Oxygen is an element in group 16 of the periodic table. Therefore, there are 6 valence electrons in the oxygen atom, so the total number of valence electrons in the O3 molecule = 3 valence electrons of the oxygen atom = 6(3) = 18.
Step 2 Identify the central atom
The central atom must have a high valence or minimal electronegativity. For the ozone molecule, all three atoms are identical, so you can choose any atom as the central atom.
Step 3 Labelling the electron lone pairs between atoms
Total valance electrons pairs = σ bonds + π bonds + lone pairs at valence shells, total electron pairs are determined by dividing the number total valence electrons by two. For O3 molecule, Total number of pairs of electrons are 9.
A central oxygen atom is connected to each of the other two oxygen atoms by a σ-bond (one σ-bond is equal to one electron pair), and the remaining seven electron pairs are distributed as follows: two pairs of electrons on the central oxygen atom, one of which carries the same pair of electrons as the central atom, and three pairs of electrons on the other oxygen atom.
Step 4 Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible.
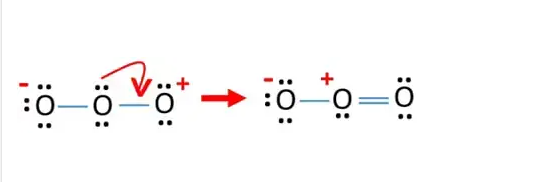
In order to check the stability of the central oxygen (O) atom we must check that it is forming an octet, if it does not have an octet then the lone pair of electrons will move to form a double or triple bond, in order for this oxygen atom to be stable the electron pairs must be moved away from the outer oxygen atom so that the oxygen atom can have eight electrons (i.e. an octet). After the move, one oxygen atom forms a double bond with the central oxygen atom, with one lone pair of electrons remaining on the central oxygen atom, and the remaining oxygen atom electron pairs remain unchanged. See the diagram below.