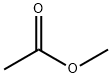
Is Methyl acetate safety
Introduction
Methyl acetate (MeAc) is a valuable and high-volume commodity chemical, and its production throughput for a typical plant ranges from 18,000 to 200,000 metric tons per year.

Uses
Methyl acetate can occur naturally in food (for example, fruits) and may be used as a flavouring agent. It is primarily used as a solvent and an intermediate feedstock in manufacturing various polymers such as photographic film base, cellulose acetate, Tenite cellulosic plastics and Estron acetate. It is also used as a solvent in adhesives, paint systems, cosmetic agents and cleaning products (approx. 70%) and as a chemical intermediate in the production of methanol, acetic acid, plant protection products and vitamins (10%); the rest (20%) is exported.
Methyl acetate is used mainly as a solvent in a variety of products. For example, it's used in:
adhesives and sealants
nail polish and remover
cleansers and exfoliants
spray paints and coatings
paint strippers or removers
Methyl acetate is also known as acetic acid methyl ester.
Toxicity
In the body, methyl acetate is hydrolyzed into methanol and acetic acid. It causes narcosis in animals exposed to high concentrations. "TLV Basis" is headache, dizziness, nausea, and damage to the ganglion cells in the retina. The main concern is conversion to methanol, which can cause permanent blindness. Eye damage has been reported after chronic exposure to methanol at 1200-8000 ppm. Ethyl acetate is in the list of "Some volatile substances which may be abused by inhalation", published on the website of the U.N. International Drug Control Programme, indicating its potential to cause narcosis in workers. An eye and respiratory tract irritant: Inhalation of high concentrations can cause CNS depression and affect the optic nerve, leading to impaired vision. See "Methanol."
Methyl acetate is a water-soluble substance with high volatility. The substance has narcotic properties if inhaled at concentrations of 34 mg/l (mice) and 56 mg/l (cats), with a short duration of the narcotic action after cessation of exposure. Methyl acetate is absorbed via the lungs in animals and humans; absorption via the oral route is demonstrated. After absorption, the substance undergoes hydrolysis to methanol and acetic acid. From the available in vitro data, it may be anticipated that the half-life of methyl acetate in blood will range between 2 and 4 hours. Immediately after stopping a 6-hour inhalation exposure to rats (2,000 ppm (6,040 mg/m3)), blood concentrations below the limit of quantification (less than 4.6 mg/l) were determined, indicating rapid hydrolysis and high clearance of the substance. It appears from these data that the systemic availability of methyl acetate is low. The main metabolite is methanol, which is metabolized by formic acid. Formate is introduced into C1-metabolism after activation by reacting with tetrahydrofolate. Humans, as well as monkeys, are more sensitive to methanol poisoning compared with rats because of a lower tetrahydrofolate content in the liver. Therefore, interspecies differences in the metabolism were considered mainly of concern at dose levels that lead to acute toxicity. Thus, rats are useful models for indicating subacute/subchronic toxic effects below sublethal dosages. Assessment of the available animal toxicology data indicates that methyl acetate is of low acute toxicity (rats LD50 oral: 6,482 mg/kg bw, dermal: >2,000 mg/kg bw, LC50 inhalation>49 mg/l/4h). After oral application and after inhalation of substance vapours, animals showed narcotic symptoms, spasms, dyspnea and vomiting; inhalation of vapours, in addition, caused irritation of the eyes and upper respiratory tract. The narcotic concentration for mice starts at 34 mg/l and for cats with 56 mg/l inhaled. In humans, accidental inhalation of vapours of methyl acetate causes severe headaches and considerable somnolence. Methyl acetate has proven to cause only weak skin irritation in humans and rabbits (no oedema, erythema with maximum grade 1 reversible within 48 hours). Eye irritation, however, was strong but reversible within 7 days of a Draize eye test with rabbits. Exposure to methyl acetate vapours causes irritation to the eyes and respiratory tract of humans. Taking into account the long experience with human exposure to the substance, methyl acetate is not supposed to exhibit skin-sensitizing properties. However, no relevant human or animal data are available.
You may like
Related articles And Qustion
See also
Lastest Price from Methyl acetate manufacturers

US $10.50/KG2025-06-06
- CAS:
- 79-20-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 ton
US $100.00/KG2025-04-21
- CAS:
- 79-20-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON