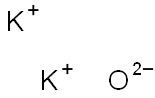Is k2o ionic or covalent?
Mar 15,2024
Potassium oxide(K2O)also called Potassium Monoxide, Di-potassium Hydroxide, and Kalium Oxide, is an ionic compound formed by combining potassium and oxygen.
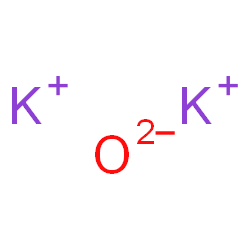
Potassium cannot be found in its natural state because it is highly reactive. It has valency +1 and combines readily with oxygen atoms forming K2O. When potassium is oxidized, Potassium Oxide, K2O, is formed as a grey crystalline substance. Potassium oxide is a strongly corrosive alkali, when dissolved in water.
Potassium Oxide is widely used as a fertilizer in agriculture.