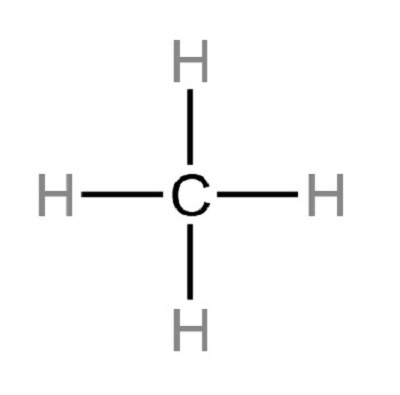
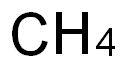Is CH4 a polar or non-polar molecule?
The CH4 molecule is a non-polar molecule because there is no net dipole moment in the molecule.
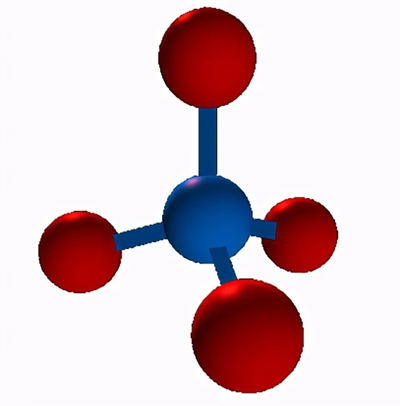
Dipole Moment
The polarity of compounds is determined by the difference in their electronegativity or the degree of charge separation in the bonds, which is called the dipole moment (μ). A molecule is considered polar if the atoms have a large difference in electronegativity and have a net dipole (i.e., the dipoles of the bonds do not cancel each other out). Conversely, the molecule is non-polar.
CH4 polarity
CH4. As we have seen, the C-H bonds in methane are polar. However, a molecule of methane is non-polar. Specifically, the dipole moment of methane is zero. A dipole moment of zero means that the "center of negative charge" in the molecule corresponds to the "center of positive charge". In the case of methane, the "center of positive charge" and the "center of negative charge" are focused on the carbon atom. Think of the "center of charge", whether positive or negative, in the same way that you think of the "center of mass". From that perspective, a molecule with a dipole moment of zero is like a perectly balanced see-saw.
Overall, the net dipole moment in a molecule is the sum of the dipole moments present in the molecule. Here, the dipole moment in the CH4 molecule is zero because all the dipole moments are cancelled out. Since there is no net dipole moment in the molecule, CH4 becomes a non-polar molecule.