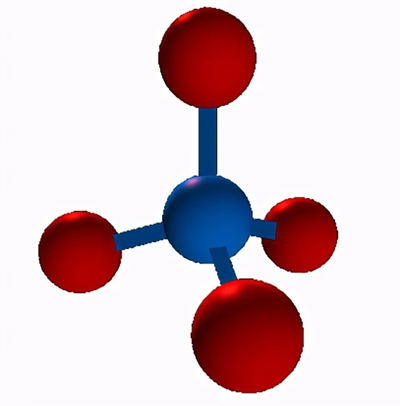
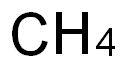The Lewis structure of CH4
What is CH4
CH4 is commonly named methane. Methane is the simplest hydrocarbon in the organic molecule, and a hydride of C. Methane is a naturally occurring gas relatively abundant on the Earth, making it an economically efficient fuel. As it releases more light and heat on burning, it is preferred over coal, fossil fuel, or gasoline for energy production. Substantive methane reserves have been discovered in recent decades, including shale gas and gas hydrates. Moreover, methane is the main constituent of biogas, a renewable resource. In the process of coal mining, a large amount of methane is emitted into the atmosphere every year, which enhances the greenhouse effect of the atmosphere[1-2].
How to draw the Lewis structure of CH4
a. Count total valence electrons in CH4. Carbon is a group IVA element in the periodic table with four electrons in its last shell. Hydrogen is a group IA element in the periodic table and contains only one electron in its last shell. Hence, the total number of valence electrons of CH4 is calculated by the following formula:
Total valence electrons = valence electrons given by 1 Carbon atom + valence electrons given by 4 hydrogen atoms = 4 + 1*(4) = 8
b. Find the least electronegative atom and place it at the center. In this case, carbon is the center atom because hydrogen always goes outside in the Lewis diagram.
c. Connect the atoms with single bonds. Simply connect each outer atom (hydrogen) to the central atom(carbon) with the help of a single bond. As shown below, the molecule has four single bonds representing four Bonding pairs. Specifically, all the valence electrons of the CH4 molecule are used for bonding.
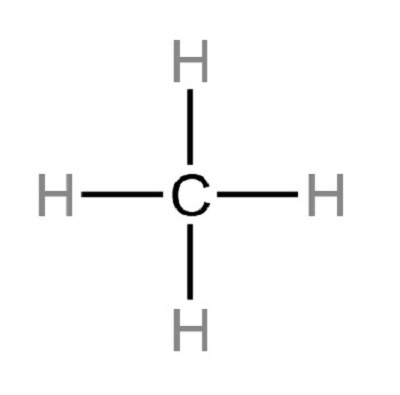
d. Check the formal charge. The lesser the formal charge on atoms, the better the stability of the Lewis diagram. To calculate the formal charge on an atom. Use the formula given below:
Formal charge = Valence Electrons – Unbonded Electrons – ½ Bonded Electrons
For hydrogen atom: Formal charge=1-0-2/2=0
For carbon atom: Formal charge=4-0-8/2=0
Hence, the overall formal charge is zero. It is also reflected that the molecule is also neutral in the CH4 Lewis structure.
References
[1] Zhengxin Xu, E. Park. "Gas-Phase Selective Oxidation of Methane into Methane Oxygenates." Catalysts (2022).
[2] Yinbo Zhou . "Characteristics of the methanotroph used in coalbed methane emission reduction: Methane oxidation efficiency and coal wettability." Fuel 349 (2023): Article 128596.