Is CH2Cl2 Polar or Nonpolar?
Dichloromethane CH2Cl2 is a polar molecule. It(CH2Cl2) consists of a central carbon (C) atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen (H) atoms and two chlorine (Cl) atoms.
The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape.The such arrangement leads to a tetrahedral geometry with a bond angle of 109.5°.
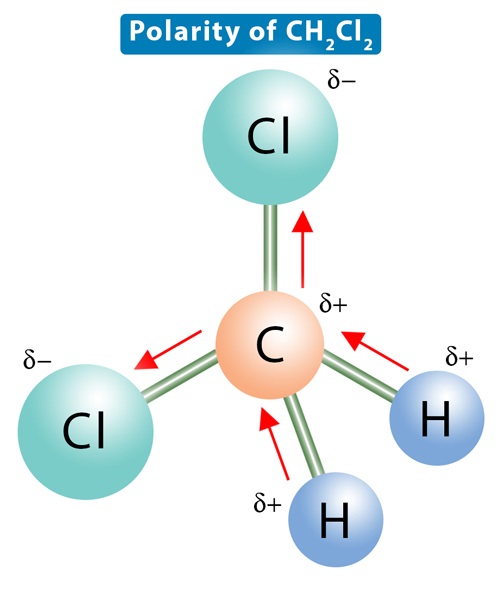
The electronegativity of carbon is 2.55, that of chlorine is 3.16, and that of hydrogen is 2.2. An electronegativity difference of 0.61 units exists between a carbon and a chlorine atom in the C-Cl bond in the CH2Cl2 molecule. Therefore, the C-Cl bonds are more polar than the C-H bonds, so there is some net residual polarity. The bond dipoles are arranged asymmetrically. There is no way to arrange the molecule such that the dipole moments cancel out. As a result, there will be a net dipole moment, making dichloromethane a polar molecule.
Related articles And Qustion
See also
Lastest Price from Dichloromethane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 75-09-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/kg2025-04-15
- CAS:
- 75-09-2
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons