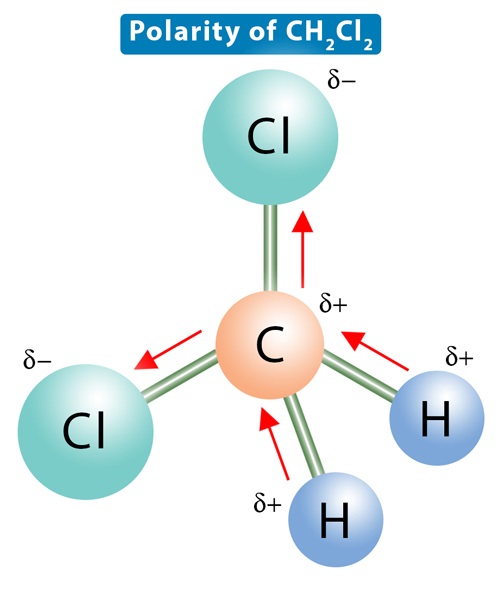
Toxicity of Dichloromethane
Dichloromethane (DCM; mol. wt. 93.328) was first prepared in 1840 by mixing chloromethane and chlorine and exposed to sunshine. It has been used as a versatile solvent to dissolve various organic compounds in many chemical processes since World War II. Currently, dichloromethane is manufactured by two sets of processes, hydrochlorination of methanol and direct chlorination of methane. Dichloromethane is considered a carcinogenic material.
DCM is primarily metabolized in the liver forming carbon monoxide (CO) and ultimately elevating blood carboxyhemoglobin levels. Carboxyhemoglobin levels may continue to rise for several hours after exposure has ceased. CO is extremely fetotoxic. Children are more vulnerable to toxic effects and their metabolites of DCM. Electrocardiographic changes resembling those of carbon monoxide poisoning are common after DCM exposure. Elevated carboxyhemoglobin levels may cause insufficient oxygen supply to the heart in persons who have preexisting coronary disease. Angina, myocardial infarction, and cardiac arrest associated with methylene chloride inhalation were reported in one patient, but no adverse cardiovascular effects from methylene chloride have been reported for occupationally exposed workers. Nausea, vomiting, gastrointestinal ulceration, and bleeding have been reported after ingestion. Liver dysfunction may result from acute, high-level exposure to methylene chloride. The National Institute for Occupational Safety and Health (NIOSH) recommends that methylene chloride be regulated as an occupational carcinogen.

Uses
Originally, dichloromethane was blended with other compounds, such as alcohols, acids, amines and paraffin, in paint strippers to be against specific coatings. From the middle of 1980s, it was applied as a component of low-temperature heat-transfer medium in the installations of air cooling systems. In some food industries, dichloromethane can also be utilized as an extraction solvent in food industries. It is used in the production of photographic films, synthetic fibers, pharmaceuticals, adhesives, inks, and printed circuit boards. It is employed as a blowing agent for polyurethane foams and as a propellant for insecticides, air fresheners, and paints.
Environmental Fate
Dichloromethane is usually released to the atmosphere. It can react withhydroxyl radicals with a half-life of about a fewmonths. Dichloromethane released to water can be evaporated to atmosphere with a half-life of 35.6 h at moderate mixing conditions. Some of dichloromethane in water can be biodegraded completely within several hours and a few days. Small part of dichloromethane released to water can be degraded by hydrolysis. However, hydrolysis is not an important process under natural condition and may take 18 months or more to degrade completely. Dichloromethane released to soil will go to the soil surface and then the atmosphere. Some part of dichloromethane in soil will leak to the groundwater and water cycle.
DCM’s production and use as solvent, chemical intermediate, grain fumigant, paint stripper and remover,metal degreaser, and refrigerant may result in its release to the environment through various waste streams. Vapor-phase DCM is expected to be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be approximately 119 days (in the absence of direct photolysis). If released to soil,DCMis expected to have very high mobility based on an estimated Koc of 24. Volatilization from moist soil surfaces is expected to be an important fate process based on an estimated Henry’s law constant of 3.25×10-3 atm-m3 mol-1. DCM may volatilize from dry soil surfaces based on its vapor pressure. Biodegradation in soil may occur. DCM, when released into water, is not expected to adsorb to suspended solids and sediment in water based on the estimated Koc. Biodegradation is possible in natural waters but will probably be very slow compared with evaporation.
Mechanism of Toxicity
The mechanism is still unclear so far. The most possible
mechanism for dichloromethane toxicity is that this
compound can increase potassium ions current in certain types
of potassium channels. The correlation between biological
activity (toxicity and mutagenic effectiveness in Salmonella TA
100) and reactivity toward strong nucleophiles indicates that
reactions with nucleophilic groups of high reactivity in biological
materials, possibly SH or amino groups in proteins, are involved in dichloromethane’s mechanism of action. Increases
in the concentration of DCM lower the oxygen affinity of
human hemoglobin. DCM binds weakly to hemoglobin at four
different sites, but binding to only one site is responsible for
decreasing the oxygen affinity of hemoglobin.
You may like
Related articles And Qustion
See also
Lastest Price from Dichloromethane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 75-09-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/kg2025-04-15
- CAS:
- 75-09-2
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons