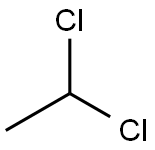
Uses and Toxicity of 1,1-Dichloroethane
Chlorinated aliphatics as a class are known to cause central nervous system (CNS) depression and respiratory tract and dermal irritation when humans are exposed by inhalation to sufficiently high concentrations. In the past, 1,1-dichloroethane was used as an anesthetic; however, this use was discontinued due to the risk of induction of cardiac arrhythmia in humans.

Uses
1,1-Dichloroethane has a number of uses: as a solvent for plastics, oils, and fats; as a cleaning agent; as a degreaser; in rubber cementing; as a fumigant and insecticide spray; in fabric spreading; in fire extinguishers; in medications; and formerly as an anesthetic. It also is an extractant for heat-sensitive substances; it is used in the manufacture of high-vacuum rubber; as a coupling agent in antiknock gasoline; as paint, varnish, and finish remover; and in organic synthesis and ore flotation. 1,1-Dichloroethane itself is usually used as an intermediate in the production of vinyl chloride and of 1,1,1-trichloroethane by thermal chlorination or photochlorination.
Environmental Fate
Production and use of 1,1-dichloroethane as a chemical intermediate, in paint removers, and as a antiknock gasoline additive may result in its release to the environment through various waste streams. If released to air, it will exist solely as a vapor in the ambient atmosphere. Vapor-phase 1,1-dichloroethane will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals (estimated half-life, 49 days). 1,1-Dichloroethane when released to soil is expected to have very high mobility based on a Koc of 30. Volatilization from moist soil surfaces is expected to be an important fate process of this compound. 1,1-Dichloroethane may volatilize from dry soil surfaces based on its vapor pressure. Halogenated aliphatic hydrocarbons are generally considered to be resistant to biodegradation. However, in water, 1,1-dichloroethane is not expected to adsorb to suspended solids and sediments based on the Koc. Estimated volatilization half-lives for a model river and model lake are 3 h and 4 days, respectively. An estimated bioconcentration factor of 5 suggests the potential for bioconcentration in aquatic organisms is low. The environmental hydrolysis half-life at 25°C and pH 7 is 61 years.
Mechanismof action
The mechanismof action of dichloroethane-induced toxicity is not fully elucidated. By most criteria, 1,1-dichloroethane is less toxic than 1,2-dichloroethane. In vivo studies of the metabolism of 1,1-dichloroethane in humans and animals are very limited. Elucidation of 1,1-dichloroethane’s metabolic scheme to date is primarily based on in vitro studies. In general, the identification of specific metabolites and the monitoring of enzyme activities indicate that the biotransformation of 1,1-dichloroethane is mediated by hepatic microsomal cytochrome P450 system.
The highest concentrations were found in fat. Following
absorption, dichloroethanes are distributed throughout the
body rapidly and are metabolized via biotransformation
process mediated through cytochrome P450 and glutathione
conjugation pathways. Following inhalation exposure in rats,
elimination occurred primarily via the excretion of soluble
metabolites and unchanged parent compound in urine and
carbon dioxide in the expired breath (less than 15%).
Urinary metabolites accounted for 84% of the absorbed dose,
fecal accounted for 2%, and carbon dioxide accounted for
~7%. Following oral exposure, urinary metabolites accounted
for 60% of the administered 150 mg kg-1 dose. A variety
of metabolized products from dichloroethanes, including
chloroacetic acid, thiodiacetic acid, and thiodiacetic acid
sulfoxide, are very reactive species and are more toxic than
the parent compound.
Toxicity
Crystal precipitations and obstruction in the renal tubule lumina and increases in serum urea and creatinine were observed in cats exposed to this compound for weeks. However, these effects were not observed in rats, guinea pigs, or rabbits. However, kidney effectswere observed inmice administered a lethal intraperitoneal injection; the effects included increased glucose and protein in the urine and tubular swelling. The toxicological significance of the nephrotoxicity observed in cats and the mice with regard to human health is not known given the small number of animals tested (cats).The detectionof 1,1-dichoroethane or itsmetabolites in blood and urine cannot predict the type of health effects that might develop from that exposure; because 1,1-dichloroethane and its metabolites leave the body fairly rapidly, the tests need to be conducted within hours to days after exposure.