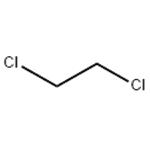
Toxicity of 1,2-Dichloroethane
1,2-Dichloroethane is a clear, manufactured liquid that is not found naturally in the environment. It evaporates quickly at room temperature and has a pleasant smell and a sweet taste. 1,2-Dichloroethane burns with a smoky flame. The most important use of 1,2-dichloroethane is to make vinyl chloride, which is used to make a variety of plastic and vinyl products, including polyvinyl chloride pipes and other important construction materials, packaging materials, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used as a solvent and is added to leaded gasoline to improve octane rating.

Uses
1,2-Dichloroethane is used as a solvent, pesticide, fumigant, gasoline additive, degreaser, and paint remover and in the synthesis of vinyl chloride monomer. 1,2-Dichloroethane is also used as a chemical intermediate in soaps and lead scavenger.
Environmental Fate
1,2-Dichloroethane can enter the environment when it is made, packaged, shipped, or used. Most 1,2-dichloroethane is released to the air, although some is released to rivers or lakes. 1,2-Dichloroethane could also enter soil, water, or air in large amounts in an accidental spill (evaporates into the air very fast from soil and water). If released to air, a vapor pressure of 78.9mmHg at 25°Cindicates that 1,2-dichloroethane will exist solely as a vapor in the ambient atmosphere. Vapor-phase 1,2-dichloroethane will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 63 days. Indirect evidence for photooxidation of 1,2-dichloroethane comes from the observation that monitoring levels are highest during the night and early morning. It may also be removed from air in rain or snow.
Since it stays in the air for a while, the wind may carry it over large distances. In water, 1,2-dichloroethane breaks down very slowly and most of it will evaporate to the air. Only very small amounts are taken up by plants and fish. Exact longevity of 1,2-dichloroethane in water remains unknown. It is thought that it remains longer in lakes than in rivers.
In soil, it either evaporates into the air or travels down through soil and enters underground water. 1,2-Dichloroethane has been found in the United States drinking water at levels ranging from 0.05 to 64 ppb. An average amount of 175 ppb has been found in 12%of the surface water and groundwater samples taken at 2783 hazardous wastes sites. 1,2-Dichloroethane has also been found in the air near urban areas at levels of 0.10–1.50 ppb and near hazardous waste sites at levels of 0.01–0.003 ppb. Small amounts of 1,2-dichloroethane have also been found in foods.
Small organisms living in soil and groundwater may transform it into other, less harmful compounds, although this happens slowly. Large amounts from an accident, hazardous waste site, or landfill may likely reach the underground and contaminate drinking water wells. Biodegradation occurs slowly in water and soil surfaces. It is not expected to undergo hydrolysis and photolysis. Humans may get exposed to very lowlevels of 1,2-dichloroethane through its use as a gasoline additive (leaded gasoline is no longer used in the United States).
Side effects
1,2-Dichloroethane is a central nervous system (CNS) depressant that produces symptoms ranging from nausea, vomiting, headache, lightheadedness, and weakness to stupor, disequilibrium, coma, and respiratory arrest. Typically, in severe cases, CNS signs appear first, followed by a quiescent period ultimately leading to oliguria and hepatic transaminasemia. These conditions may occasionally proceed to hepatorenal failure. Severe ingestions produce widespread organ injury (especially kidney, liver, and adrenal gland) as well as gastrointestinal bleeding.
Toxicity
The mechanism of action of dichloroethane-induced toxicity
is not fully elucidated. Toxicity and mutagenicity of
1,2-dichloroethane are supported by various genotoxicity
assays in various models. Direct evidence of 1,2-dichloroethane
genotoxicity was shown in a number of in vitro and in vivo
studies measuring effects such as mutations, sister chromatid
exchange frequency, recombination, micronuclei induction,
DNA-strand breaks, and DNA adducts. The 1,2-dichloroethane
data set encompasses multiple end points and routes of
exposure as well as activation via multiple metabolic pathways,
including hepatic glutathione transferases and microsomal
cytochrome P450 enzymes.
You may like
Related articles And Qustion
See also
Lastest Price from 1,2-Dichloroethane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 107-06-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00/kg2025-04-15
- CAS:
- 107-06-2
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons



