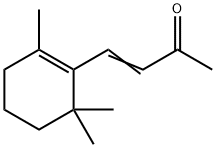
Irisone: Biological Functions, Metabolic Engineering, and Chemical Synthesis Routes
Irisone is a natural plant volatile compound, and it is the 9,10 and 9',10' cleavage product of it by the carotenoid cleavage dioxygenase. It is widely distributed in flowers, fruits, and vegetables. Irisone and other apocarotenoids comprise flavors, aromas, pigments, growth regulators, and defense compounds; serve as ecological cues; have roles as insect attractants or repellants, and have antibacterial and fungicidal properties. In recent years, Irisone has also received increased attention from the biomedical community for its potential as an anticancer treatment and for other human health benefits. However, Irisone is typically produced at relatively low levels in plants. Thus, expressing plant biosynthetic pathway genes in microbial hosts and engineering the metabolic pathway/host to increase metabolite production is an appealing alternative.

Occurrence and Biological Function and Metabolic Engineering
Plant volatile organic compounds (VOCs) are specialized metabolites, which have been described from more than 90 plant families . A wide diversity of compounds are emitted from leaves, flowers, fruits, bark, and roots, as well as from specialized storage structures, e.g., glands. VOCs are lipophilic liquids and have low molecular mass (<300 Da) with high vapor pressures. Plant VOCs are derived from various biosynthesis pathways and are often classified into significant groups, including phenylpropanoids, fatty acids, amino acids, and terpenoids. The name “ionone” is derived from “iona” (Greek for violet), which refers to the violet odor, and “ketone,” which refers to the structure of the ionone. Irisone is the 9,10 and 9′,10′ cleavage product of β-carotene. It is a common aromatic volatile found in various tissues in many plant species, e.g., in the essential oil (EO) of Osmanthus fragrans, Petunia hybrid, and Rosa bourboniana, and in fruits and vegetables, including carrot, tomato, melon, raspberry, apricot, plum and apple. Irisone has become quite attractive for commercial applications and in medical and pharmaceutical industries. Plant apocarotenoids, including β-ionone, comprise flavors, aromas, pigments, growth regulators, and defense compounds. They serve as ecological cues, have roles as insect attractants or repellants, and possess antibacterial and fungicidal properties. Irisone and other apocarotenoid pigments are also economically important because they show extremely potent odor thresholds, at a level of 0.007 ppb.[1]
Currently, the production of Irisone is mainly dependent on chemical synthesis. The extraction of β-ionone from various plant species is limited, as it is for several other plant natural compounds, by low recovery rates and the complexity of the mixtures obtained from plants. Furthermore, the total chemical synthesis of Irisone is economically impractical. As a result, one appealing alternative is to express the specific plant biosynthetic pathway genes in microbial hosts such as Saccharomyces cerevisiae or Escherichia coli, and then engineer the metabolic pathway/host to improve metabolite production. The expression of heterologous genes using high-copy-number plasmids is a widely used approach for achieving high titers of secondary metabolites in biofactories. In conclusion, promising benefits for agriculture and humans have been reported for the apocarotenoid Irisone, extracted from various plant species. Although several studies proved that it possesses biological activity as an insect attractant/repellant volatile organic compound, further studies are recommended to investigate the biological activity aspect of insects.
Synthesis of Irisone
Violet scent (ionone) is currently used in many commercial products as its price is low. However, before the 1890s violet flower oil was considered the most precious of all essential oils. This is because the production of one kilogram of violet oil requires 33,000 kg of violet flowers, which cost approximately 82,500 German gold marks for raw materials. Thus in 1893, Tiemann and Krueger investigated the compound responsible for the scent of violets by studying orris root oil; a much cheaper oil with a similar odour principle. They achieved the synthesis of ionone in two steps. First, they performed an aldol condensation of citral with acetone (dimethyl ketone) in an alkaline medium to produce pseudoionone. Then pseudoionone was cyclized to ionone upon exposure to acidic conditions. The type and concentration of acid used determines the ratio of α-ionone and Irisone produced in this reaction. For example, phosphoric, fumaric and other weaker acids mainly yield α-ionone while concentrated sulfuric acid preferentially yields Irisone. This synthesis marked the start of fragrance chemistry (Duftstoff-Chemie). Today the global usage of α-ionone and β-ionone is approximately between 100 and 1000 metric tons yearly.[2]
The association between the level of Irisone and BCO2 mutations has not been investigated. It appears likely that mutations or absence of BCO2 would result in reduced levels of β-ionone and might contribute in the pathogenesis of the mentioned diseases. In addition, the discovery of endogenous production of Irisone indicates that it might have physiological roles that are yet to be revealed. This is supported by the fact that high intake of fruits and vegetables, which contain volatile isoprenoids such as ionones, is associated with lower risk of cancer. Studies have focused to understand the biological role of apocarotenoids generated by BCO2, β-ionone has been less studied. The turpentine derived from the resin of terbenith tree had been widely used as a medicine and in wine as a preservative agent and taste enhancer. It was noticed that the consumption of turpentine oil alters the urine scent into violet. Anecdotally, turpentine oil was allegedly used by Cleopatra for this purpose. Al-Tel and his colleagues suggested a possible metabolic pathway for the conversion of α-and β-pinene into α-and Irisone. First, the CYP enzyme would oxidize α-and β-pinene generating intermediates, respectively. The intermediate generated from β-pinene will undergo free radical rearrangement forming intermediate. The latter will abstract a hydrogen radical from CYP to yield intermediate.
References
[1]Paparella A, Shaltiel-Harpaza L, Ibdah M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants (Basel). 2021 Apr 12;10(4):754. doi: 10.3390/plants10040754. PMID: 33921545; PMCID: PMC8069406.
[2]Aloum L, Alefishat E, Adem A, Petroianu G. Ionone Is More than a Violet's Fragrance: A Review. Molecules. 2020 Dec 10;25(24):5822. doi: 10.3390/molecules25245822. PMID: 33321809; PMCID: PMC7764282.
You may like
Lastest Price from Irisone manufacturers
US $0.00-0.00/kg2025-08-20
- CAS:
- 14901-07-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/KG2025-05-07
- CAS:
- 14901-07-6
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS