Introduction, Synthesis, and Pharmacokinetics of Oseltamivir Phosphate
General description
Oseltamivir phosphate is an anti-flu drug marketed as Tamiflu. Appearing as A white to yellowish-white powder, it can be classified as selective inhibitors of influenza neuraminidase (type-A and B), which control influenza symptoms by inhibiting the neuraminidase of the virus and preventing its release and spread from infected cells [1]. Clinically, oseltamivir phosphate is used for the treatment of influenza A and B in adults and children 1 year and older, and for the prevention of influenza A and B in adults and adolescents (13 years and older). It is rapidly metabolized into oseltamivir carboxylate after absorption by the gastrointestinal tract, and at least 75% of the oral dose is circulated in the system as oseltamivir carboxylate [2]. The active ingredient is a powerful and unique neuraminidase inhibitor that acts at all stages of influenza virus infection and prevents replication of all clinically relevant influenza A or B strains. Inhibitory effects are observed at very low nanogram concentrations in vitro. The active metabolites are observed to inhibit influenza virus growth in vitro and replication and pathogenicity of influenza virus in vivo. This product reduces transmission of influenza A or B virus by inhibiting the release of the virus from infected cells [3].
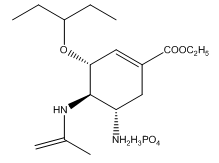
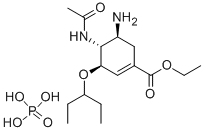
Fig. 1 The structure of oseltamivir phosphate.
Synthesis
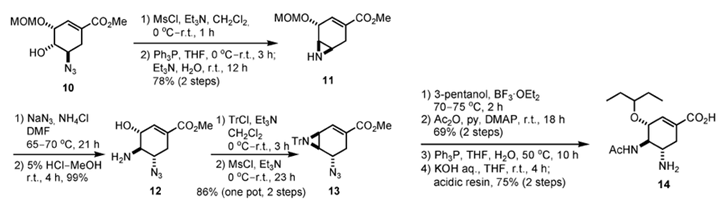
Fig. 2 The synthetic route of oseltamivir phosphate [4].
The medicinal chemistry group at Gilead Sciences first synthesized 6 from a natural product, (–)-shikimic acid, as the starting material. Treatment of (–)-methyl shikimate (8) under Mitsunobu conditions produced anepoxide through selective activation of the least sterically hindered hydroxy group at C-5. After the remaining hydroxy group at C-3 had been protected with a MOM group (9), epoxide ring-opening with azide proceeded selectively at the sterically less hindered C-5, affording azido alcohol 10. O-Mesylation, followed by a Staudinger reaction to generate iminophosphorane, aziridinium formation through substitution of the mesyl group, and hydrolysis of the intermediate with Et3N in H2O, produced aziridine 11. Regioselective aziridine-opening with azide proceeded at C-5, and cleavage of the MOM group afforded amino alcohol 12.
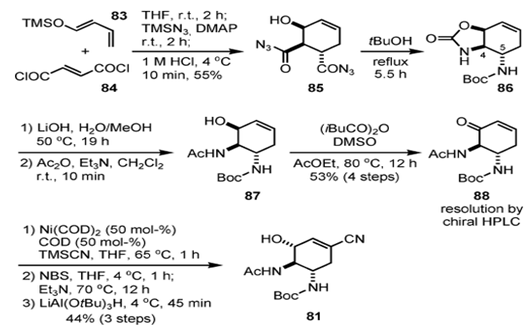
Fig. 3 The synthetic route of oseltamivir phosphate [5].
A completely different synthetic route using the Diels–Alderreaction and Curtius rearrangement as key steps.26 Although the third-generation route depended on resolution by chiral HPLC, it required significantly fewer steps (12 steps) than the first- and second-generation syntheses. The Diels–Alder reaction between siloxy diene 83 and fumaroyl chloride (84) proceeded at room temperature over 2 h. After the reaction was complete, TMSN3 and DMAP were added to the mixture, and the corresponding acylazide was formed. On quenching of the reaction with HCl (1 M), desilylation took place and 85 was obtained in 55 % yield over three operations. Although the Diels–Alder reaction afforded a 2:1 (endo/exo) mixture of diastereomers, the undesired exo isomer selectively decomposed during the acidic cleavage of the TMS ether. A Curtius rearrangement was conducted by heating a tBuOH solution of acyl azide 85 at reflux. In this process, two nitrogen atoms at C-4 and C-5 were differentiated, affording 86. Selective hydrolysis of the cyclic carbonate with LiOH and subsequent N-acetylation produced 87, which was oxidized to enone 88 under modified Moffat conditions with isobutyric anhydride.
Pharmacology and toxicology
Oseltamivir phosphate is a pharmaceutical precursor of its active metabolite, which is a potent selective influenza virus neuraminidase inhibitor. Viral neuraminidase activity is critical for the release of newly formed virus particles from infected cells and for further spread of infectious viruses in humans [6].
In the phase III clinical trial, the patient began to treat with oseltamivir phosphate after the onset of clinical symptoms for no more than 60 hours. This treatment significantly shortened the duration of influenza symptoms and signs, up to 45 hours. Compared with placebo, oseltamivir phosphate can reduce the severity of influenza by about 40%. More importantly, oseltamivir phosphate reduced the incidence of complications associated with antibiotic treatment of influenza by 50% in healthy young adults and 75% in the elderly. These complications include tracheitis, pneumonia, and sinusitis [7].
Studies on naturally acquired and laboratory acquired influenza showed that the application of oseltamivir phosphate did not impair human normal antibody response to infection. The antibody response of subjects to inactivated influenza vaccine was not affected by oseltamivir phosphate.
The possibility of drug resistance of the virus was deeply studied [8]. In clinical isolates, the incidence of drug resistance depends on the subtype, which is about 2%. Drug resistant virus carriers can clear the virus as normal, and there is no sign of deterioration in clinical practice. Drug resistant genotypes have no advantage, and their infectivity to humans is reduced [9].
Pharmacokinetics
(1) Absorption
After oral administration, oseltamivir is easily absorbed by the gastrointestinal tract, and most of it is converted into active metabolites by liver and intestinal esterases. At least 75% of the oral dose enters the systemic circulation in the form of active metabolites. Compared with active metabolites, the exposure of drug precursors is less than 5%. The plasma concentration of drug precursors and their metabolites is proportional to the dosage and is not affected by food intake [10].
(2) Distribution
The average volume of distribution (VSS) of active metabolites is about 23 liters in human body. Studies on ferrets, rats and rabbits have shown that the active part of the drug can reach all target tissues invaded by influenza virus. At the same time, the study also confirmed that after oral administration of oseltamivir phosphate, its active metabolites accumulated in the lung, trachea, bronchoalveolar lavage fluid, nasal mucosa and middle ear. The binding of active generation products to human plasma proteins is negligible (about 3%) [11].
(3) Metabolize
Oseltamivir phosphate is mostly converted into active metabolites by esterases located in the liver and intestine. Oseltamivir phosphate or its active metabolites are not substrates or inhibitors of major cytochrome isoenzymes. Therefore, it is unlikely that drug interactions will be triggered by competitive inhibition of these enzymes [12].
(4) Elimination
Oseltamivir phosphate absorbed is mainly (> 90%) cleared by conversion to active metabolites. Active metabolites are no longer further metabolized, but excreted by urine. In most subjects, the peak plasma concentration of active metabolites decreased with a half-life of 6-10 hours. More than 99% of the active metabolites are excreted by the kidney. The clearance rate of kidney (18.8l/h) exceeds the glomerular filtration rate (7.5l/h), indicating that in addition to glomerular filtration, there is also the pathway of renal tubular excretion. After oral administration of radiolabeled drugs, only less than 20% of the dose is excreted by feces [13].
Adverse reactions
The main adverse reactions of oseltamivir phosphate are gastrointestinal discomfort, including nausea, vomiting, diarrhea, abdominal pain, etc. Then there are adverse reactions of the respiratory system, including bronchitis, cough, etc. in addition, there are adverse reactions of the central nervous system, such as dizziness, headache, insomnia, fatigue, etc [14].
Pharmacokinetics of special population
(1) Patients with renal insufficiency
100mg oseltamivir phosphate was given to patients with different degrees of renal insufficiency twice a day for five days, which showed that the level of active metabolites was inversely proportional to the reduction of renal function. For patients with creatinine clearance rate less than 30ml/min, dose adjustment is recommended. At present, there is no research data to guide the medication of patients with renal failure (creatinine clearance is less than 10ml/nin), so we should be careful when using drugs for this population [15].
(2) Patients with liver insufficiency
After oral oseltamivir phosphate, patients with liver dysfunction did not have the expected increase in oseltamivir levels or decrease in the level of its active metabolites [16].
(3) The elderly
Given the same dose of oseltamivir phosphate, compared with young people, the steady-state metabolite level of the elderly (aged 65-78 years) is 25-35% higher than that of young people, and the drug half-life of the two populations is very similar [17, 18]. Considering the drug exposure and tolerance, the elderly do not need to adjust the dose.
References
[1] S. Abrecht, M.C. Federspiel, H. Estermann, R. Fischer, M. Karpf, H.-J. Mair, T. Oberhauser, G. Rimmler, R. Trussardi, U. Zutter, The synthetic-technical development of oseltamivir phosphate Tamiflu™: a race against time, CHIMIA International Journal for Chemistry 61(3) (2007) 93-99.
[2] S. Abrecht, P. Harrington, H. Iding, M. Karpf, R. Trussardi, B. Wirz, U. Zutter, The synthetic development of the anti-influenza neuraminidase inhibitor oseltamivir phosphate (Tamiflu®): a challenge for synthesis & process research, CHIMIA International Journal for Chemistry 58(9) (2004) 621-629.
[3] M. Federspiel, R. Fischer, M. Hennig, H.-J. Mair, T. Oberhauser, G. Rimmler, T. Albiez, J. Bruhin, H. Estermann, C. Gandert, Industrial synthesis of the key precursor in the synthesis of the anti-influenza drug oseltamivir phosphate (Ro 64-0796/002, GS-4104-02): Ethyl (3 R, 4 S, 5 S)-4, 5-epoxy-3-(1-ethyl-propoxy)-cyclohex-1-ene-1-carboxylate, Organic Process Research & Development 3(4) (1999) 266-274.
[4] G.C. Ghosh, N. Nakada, N. Yamashita, H. Tanaka, Oseltamivir carboxylate, the active metabolite of oseltamivir phosphate (Tamiflu), detected in sewage discharge and river water in Japan, Environmental Health Perspectives 118(1) (2010) 103-107.
[5] P.J. Harrington, J.D. Brown, T. Foderaro, R.C. Hughes, Research and development of a second-generation process for oseltamivir phosphate, prodrug for a neuraminidase inhibitor, Organic process research & development 8(1) (2004) 86-91.
[6] J. Ives, J. Carr, D. Mendel, C. Tai, R. Lambkin, L. Kelly, J. Oxford, F. Hayden, N. Roberts, The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo, Antiviral research 55(2) (2002) 307-317.
[7] H. Kakeya, M. Seki, K. Izumikawa, K. Kosai, Y. Morinaga, S. Kurihara, S. Nakamura, Y. Imamura, T. Miyazaki, M. Tsukamoto, Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study, PLoS One 9(3) (2014) e91293.
[8] M. Karpf, R. Trussardi, Efficient Access to Oseltamivir Phosphate (Tamiflu) via the O‐Trimesylate of Shikimic Acid Ethyl Ester, Angewandte Chemie International Edition 48(31) (2009) 5760-5762.
[9] I.A. Leneva, N. Roberts, E.A. Govorkova, O.G. Goloubeva, R.G. Webster, The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses, Antiviral research 48(2) (2000) 101-115.
[10] J. Magano, Synthetic approaches to the neuraminidase inhibitors zanamivir (Relenza) and oseltamivir phosphate (Tamiflu) for the treatment of influenza, Chemical reviews 109(9) (2009) 4398-4438.
[11] L.-D. Nie, X.-X. Shi, K.H. Ko, W.-D. Lu, A short and practical synthesis of oseltamivir phosphate (Tamiflu) from (−)-shikimic acid, The Journal of Organic Chemistry 74(10) (2009) 3970-3973.
[12] N. Satoh, T. Akiba, S. Yokoshima, T. Fukuyama, A practical synthesis of (−)‐Oseltamivir, Angewandte Chemie International Edition 46(30) (2007) 5734-5736.
[13] H. Shaim, P. McCaffrey, J.A. Trieu, A. DeAnda, S.G. Yates, Evaluating the effects of oseltamivir phosphate on platelet counts: a retrospective review, Platelets 31(8) (2020) 1080-1084.
[14] L. Shao, Y. Wu, H. Zhou, P. Qin, H. Ni, J. Peng, M. Hou, Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody, Platelets 26(5) (2015) 495-497.
[15] M. Shibasaki, M. Kanai, Synthetic strategies for oseltamivir phosphate, European Journal of Organic Chemistry 2008(11) (2008) 1839-1850.
[16] M. Shibasaki, M. Kanai, K. Yamatsugu, Recent development in synthetic strategies for oseltamivir phosphate, Israel Journal of Chemistry 51(3‐4) (2011) 316-328.
[17] R. Takanami, H. Ozaki, R.R. Giri, S. Taniguchi, S. Hayashi, Detection of antiviral drugs oseltamivir phosphate and oseltamivir carboxylate in Neya River, Osaka, Japan, Journal of Water and Environment Technology 8(4) (2010) 363-372.
[18] U. Zutter, H. Iding, P. Spurr, B. Wirz, New, efficient synthesis of oseltamivir phosphate (Tamiflu) via enzymatic desymmetrization of a meso-1, 3-cyclohexanedicarboxylic acid diester, The Journal of organic chemistry 73(13) (2008) 4895-4902.
Related articles And Qustion
See also
Lastest Price from Oseltamivir phosphate manufacturers
US $0.00/kg2025-11-25
- CAS:
- 204255-11-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/kg2025-07-21
- CAS:
- 204255-11-8
- Min. Order:
- 1kg
- Purity:
- 98.5%-102.0%
- Supply Ability:
- 10KGS