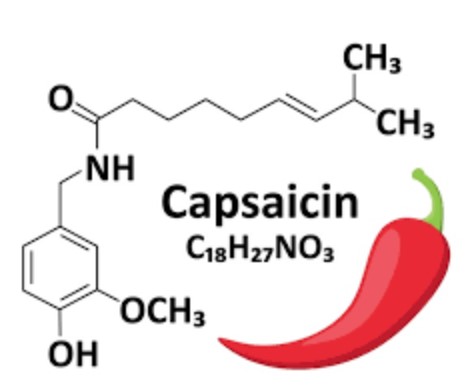
Description, Synthesis and Application of Capsaicin
General Description
Capsaicin is a unique alkaloid found primarily in the fruit of the Capsicum genus and is what provides its spicy flavor. Generally extracted directly from fruit, high demand has driven the use of established methods to increase production through extraction and characterization [1]. Over time these methods have improved, usually be applying existing techniques in conjunction. An increasingly wide range of potential applications has increased interest in capsaicin. Especially compelling are the promising results of medical studies showing possible beneficial effects in many diseases. Capsaicin’s pungency has limited its use in clinical trials to support its biological activity. Characterization and extraction/ synthesis of non-pungent analogues is in progress [2].
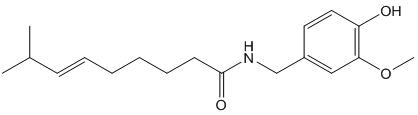
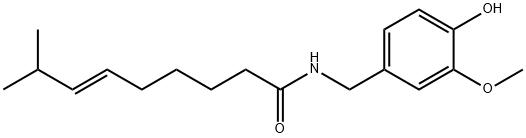
Fig. 1 The structure of capsaicin.
Compounds known as capsaicinoids cause the spicy flavor (pungency) of chili pepper fruit. The primary capsaicinoid in chili pepper is capsaicin, followed by dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin and homocapsaicin. Capsaicin and dihydrocapsaicin account for approximately 90% of capsaicinoids in chili pepper fruit, are the two most potent capsaicinoids and their molecules differ only in the saturation of the acyl group [3]. Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) is a crystalline, lipophilic, colorless and odorless alkaloid with the molecular formula C18H27NO3. Its molecular weight is 305.40 g/mol, and it is fat-, alcohol- and oil-soluble. Structure-activity relationships (SAR) for capsaicin agonists (a substance that is capable of binding to a receptor and elicit a response in the cell), have previously been rationalized by dividing the capsaicin molecule into three regions: A (aromatic ring), B (amide bond) and C (hydrophobic side chain) (Fig. 1) [4]. It is known that the substituents in the positions 3 and 4 of the A-ring are essential for potent agonist activity, and the phenolic 4-OH group in capsaicin analogues is of particular importance, H-bond donor/acceptor properties of the phenol group are key for agonist activity [5].
Capsaicin Production and Synthesis
(1) Genetic Regulation of Capsaicin Synthesis
Given the economic and agricultural importance of capsaicin, surprisingly little data exists on the genetics of this compound’s biosynthesis pathway. It is known that chili genotypes exhibit wide variation in capsaicin accumulation in response to genetic and environmental factors. The first genetic analysis of capsaicin accumulation was done by molecular mapping and revealed the presence of a Quantitative Trait Locus (QTL) called cap that may contribute to increasing pungency level [6, 7]. Most of the isolated genes related to capsaicinoids are involved in their biosynthesis, and little is known about the location and action of specific genes controlling capsaicin accumulation.
(2) Capsaicin Biosynthesis in Plants
Capsaicin biosynthesis in plants is defined by two pathways: phenylpropanoid, which determines phenolic structure; and fatty acid metabolism, which determines the molecule’s fatty acids. Capsaicin concentration increases gradually during fruit development reaching maximum levels at 40 to 50 days, after which it tends to degrade into secondary compounds due to peroxidase action. Research aimed at increasing or improving pungent compound production has revealed that hydric stress increases capsaicinoid levels because water deficit affects the phenylpropanoid pathway [8]. Hydric stress also increases capsaicin levels by raising activity of the enzymes phenylalanine ammonia-lyase (PAL), cinnamic acid-4-hydroxylase (C4H) and CS, all involved in capsaicin biosynthesis. Administration of the capsaicin precursors 8-methyl-noneic acid and vanillylamine had demonstrated that 8-methylnoneic acid occurs at lower levels than vanillylamine and is therefore the limiting substrate for capsaicin synthesis. These results suggest the possibility of controlling capsaicin synthesis in the plant by manipulating substrate concentrations and water availability, which would be a cost-effective, viable alternative for increasing capsaicin production [9].
(3) Chemical and Enzymatic Synthesis
Interest in capsaicinoids has increased in response to their multiple properties, mainly those with potential medical applications. However, clinical application of capsaicin is limited because of it pungent properties. In response, greater research effort has been applied to synthesizing natural capsaicinoids as well as synthetics with properties analogous to natural types. Enzyme-catalyzed synthesis has advantages over chemical synthesis in that it can be done using non-toxic reagents and substrate specificity. Kobata and colleagues did a number of studies involving synthesis of capsaicin analogues using amidation reactions catalyzed with different lipases [10]. In these trials, vanillylamine condensed with fatty acid derivatives, used as a substrate in the oleose phase, resulted in 40-59% capsaicin yields and synthesis of various analogues at 2–44% yields and with from 4–18 carbons. This same research group used natural oils as substrates in different reaction media (e.g. n-hexane) to synthesized non-pungent analogues.
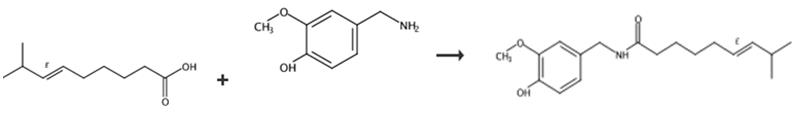
Fig. 2 The synthetic route of capsaicin [11].
4-Hydroxy-3-methoxy benzylamine HCl salt 6 (3.35 g) and dimethylformamide (10 ml) were added to a 100 ml 3-necked RB flask equipped with an additional funnel, a thermometer and a magnetic stirrer under nitrogen. 5N NaOH (7 ml) was added portion-wise at room temperature. The mixture was stirred at 35° C for 30 minutes. The mixture was then cooled to about 0°C to about 5° C Acid chloride 5 in anhydrous ether (30 ml) was added dropwise at about 0°C to about 5° C or 10° C for about 20 minutes. An additional 5 ml of anhydrous dimethylformamide was added. The mixture was gradually warmed to room temperature and stirred under nitrogen overnight. Water (150 ml) was added. The mixture was extracted with ethyl acetate (1×100 ml and 1×50 ml). The ethyl acetate extract was washed with 1N HCl (2×60 ml) followed by saturated NaHCO3 (2×100 ml) and brine. The extract was then dried over anhydrous Na2SO4 and filtered. Solvents were removed under vacuum at about 35°C to about 40°C to give a thick light orange/pink residue (wt.=3.4 g). The crude product was purified by column chromatography using from about 150 to about 160 g of silica gel eluted with a 1:1 mixture of ethyl acetate/hexane. Collections from the column purification were combined, concentrated under vacuum at about 40°C and dried under vacuum. 3.1 g of the desired product as a white solid.
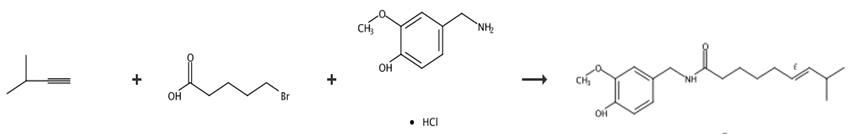
Fig. 3 The synthetic route of capsaicin [12].
4-Hydroxy-3-methoxybenzylamme hydrochloride (245 g) and dimethylformamide (700 ml) were added to a 12 L 4-necked KB flask equipped with a mechanical stirrers additional funnel and thermometer under argon. The suspension was then cooled to 10°C and 5N sodium hydroxide (494 ml) was added drop wise at temperature below 20°C. The solution was cooled to about 10°C and stirred for 30 min and then cooled to about 5°C. A solution of acid chloride (249 g) in ether (1.7 L) was added dropwise at about 5°C. The reaction mixture was gradually warmed to room temperature and stirred overnight. The reaction mixture was partitioned between 1N HCl (5L) and ethyl acetate (5L). The aqueous layer was separated and extracted with ethyl acetate (5L). The organic layers were combined, washed with 1N HCl (5L), saturated sodium bicarbonate (3x5L) and brine (5L) and then dried over sodium sulfate and filtered. Solvent was removed under vacuum to produce a crude product. The crude product was purified by two recrystallization from ether/hexane (1:2) to give 237 g (66% yield) of the desired product. HPLC confirmed a ratio of trans/cis (E/Z) of about 98:0.9 Example IV(d), yield 237 g (66%).
Capsaicin Characterization
Technological advances in the use of HPLC for organic compound analysis have been applied to capsaicin characterization, specifically liquid chromatography-mass spectrometry (LC-MS) and liquid chromatography-tandem mass spectrometry (LC-MS-MS) [13]. Capsaicin, dihydrocapsaicin and nonivamide were identified in the sprays within a 0.7 to 40.5 mg/mL interval. In other applications, LC-MS-MS has been used to quantify capsaicin, dihydrocapsaicin and nonivamide in the blood and tissues of rats exposed to inhalations of these three compounds with resulting capsaicin concentrations of 1.0 to 90.4 ng/mL in blood, 5.0 to 167 pg/mg in lungs, and 2.0 to 3.4 pg/mg in liver. Simultaneous quantification of capsaicin and dihydrocapsaicin in extracts from different Capsicum genotypes has been accomplished using liquid chromatography-electrospray/time-of-flight mass spectrometry with detection limits of 6 ng for capsaicin and 1.2 ng for dihydrocapsaicin [14].
Clinical Applications in Humans
Capsaicin has been attributed pharmacological effects since ancient times, but not until the past twenty years has extensive research been done to determine specific applications, including in the gastrointestinal tract, for weight-loss and as an analgesic [15]. capsaicin has been included in topical treatments aimed at relief of different neuropathic pain conditions, although it can produce skin irritation. Capsaicin and its analogues have been used in topical creams and patches to treat chronic pain syndromes such as post-herpetic neuralgia, musculoskeletal pain, diabetic neuropathy, osteoarthritis and rheumatoid arthritis [16]. It has also been applied to treat pain from rashes, psoriasis, mastectomy, and bladder disorders. Adverse effects (burning, stinging and erythema) are normally limited to the application site, although respiratory irritations from cream inhalation and occasional systemic effects have been reported [17]
References
[1] D. Bennett, G. Kirby, Constitution and biosynthesis of capsaicin, Journal of the Chemical Society C: Organic (1968) 442-446.
[2] S. Bevan, S. Hothi, G. Hughes, I. James, H. Rang, K. Shah, C. Walpole, J. Yeats, Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin, British journal of pharmacology 107(2) (1992) 544-552.
[3] K. Bley, G. Boorman, B. Mohammad, D. McKenzie, S. Babbar, A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin, Toxicologic pathology 40(6) (2012) 847-873.
[4] A.M. Bode, Z. Dong, The Two Faces of CapsaicinIs Capsaicin a Cocarcinogen or a Cancer Prevention Agent?, Cancer research 71(8) (2011) 2809-2814.
[5] G.A. Cordell, O.E. Araujo, Capsaicin: identification, nomenclature, and pharmacotherapy, Annals of Pharmacotherapy 27(3) (1993) 330-336.
[6] M. Fitzgerald, Capsaicin and sensory neurones—a review, Pain 15(1-4) (1983) 109-130.
[7] M. Hayman, P.C. Kam, Capsaicin: A review of its pharmacology and clinical applications, Current Anaesthesia & Critical Care 19(5-6) (2008) 338-343.
[8] S.W. Hwang, H. Cho, J. Kwak, S.-Y. Lee, C.-J. Kang, J. Jung, S. Cho, K.H. Min, Y.-G. Suh, D. Kim, Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances, Proceedings of the National Academy of Sciences 97(11) (2000) 6155-6160.
[9] J. O'Neill, C. Brock, A.E. Olesen, T. Andresen, M. Nilsson, A.H. Dickenson, Unravelling the mystery of capsaicin: a tool to understand and treat pain, Pharmacological reviews 64(4) (2012) 939-971.
[10] B. Prasad, R. Shrivastava, G.A. Ravishankar, Capsaicin, Evidence-Based Integrative Medicine 2(3) (2005) 147-166.
[11] C. Rains, H.M. Bryson, Topical capsaicin, Drugs & aging 7(4) (1995) 317-328.
[12] M.d.L. Reyes-Escogido, E.G. Gonzalez-Mondragon, E. Vazquez-Tzompantzi, Chemical and pharmacological aspects of capsaicin, Molecules 16(2) (2011) 1253-1270.
[13] S.K. Sharma, A.S. Vij, M. Sharma, Mechanisms and clinical uses of capsaicin, European journal of pharmacology 720(1-3) (2013) 55-62.
[14] A. Szallasi, P.M. Blumberg, Vanilloid (Capsaicin) receptors and mechanisms, Pharmacological reviews 51(2) (1999) 159-212.
[15] J. Szolcsányi, Forty years in capsaicin research for sensory pharmacology and physiology, Neuropeptides 38(6) (2004) 377-384.
[16] J. Winter, S. Bevan, E. Campbell, Capsaicin and pain mechanisms, British journal of anaesthesia 75(2) (1995) 157-168.
[17] W. Zhang, The effectiveness of topically applied capsaicin. A meta-analysis, European journal of clinical pharmacology 46(6) (1994) 517-522.
Related articles And Qustion
See also
Lastest Price from Capsaicin manufacturers

US $0.00-0.00/kg2025-07-07
- CAS:
- 404-86-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $5.00-0.50/KG2025-05-08
- CAS:
- 404-86-4
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS