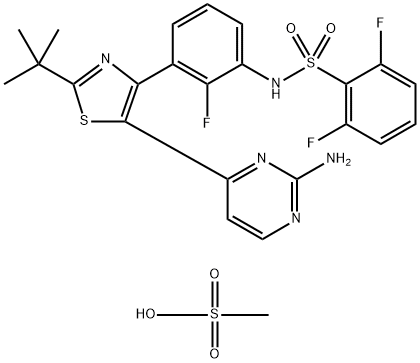
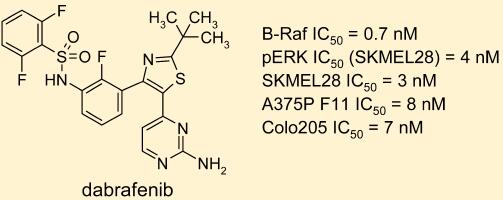
Dabrafenib Mesylate(GSK-2118436B): A Potent Inhibitor in Targeted Cancer Therapy
Dabrafenib Mesylate(GSK-2118436B) is a kinase inhibitor indicated as a single agent for the treatment of patients with unresectable or metastatic melanoma with BRAF V600E mutation as detected by an FDA-approved test.

Mechanism of action
Dabrafenib is an inhibitor of some mutated forms of BRAF kinases with in vitro IC50 values of 0.65, 0.5, and 1.84 nM for BRAF V600E, BRAF V600K, and BRAF V600D enzymes, respectively. Dabrafenib also inhibits wild-type BRAF and CRAF kinases with IC50 values of 3.2 and 5.0 nM, respectively, and other kinases, such as SIK1, NEK11, and LIMK1 at higher concentrations. Some mutations in the BRAF gene, including those that result in BRAF V600E, can result in constitutively activated BRAF kinases that may stimulate tumor cell growth. Dabrafenib inhibits cell growth of various BRAF V600 mutation positive tumors in vitro and in vivo.
Uses
Dabrafenib mesylate(GSK-2118436B) is approved to be used alone or with trametinib dimethyl sulfoxide to treat patients whose cancer has a certain mutation in the BRAF gene, including:
Anaplastic thyroid cancer that is locally advanced or has spread to other parts of the body and cannot be treated with local therapy. It is used with trametinib dimethyl sulfoxide.
Glioma that is low grade in children aged 1 year and older who require systemic therapy. It is used with trametinib dimethyl sulfoxide.
Adverse reactions
The most common side effects include papilloma (warts), headache, nausea, vomiting, hyperkeratosis (thickening and toughening of the skin), hair loss, rash, joint pain, fever and tiredness.When taken in combination with trametinib, the most common side effects include fever, tiredness, nausea, chills, headache, diarrhea, vomiting, joint pain and rash.1
Clinical research
Dabrafenib Mesylate(GSK-2118436B) has clinical activity with a manageable safety profile in clinical trials of phase I and II in patients with BRAF (V600)-mutated metastatic melanoma.
Reference
1."Tafinlar EPAR". European Medicines Agency (EMA).
You may like
Related articles And Qustion
Lastest Price from Dabrafenib Mesylate manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 1195768-06-9
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month
US $0.00/g2025-04-21
- CAS:
- 1195768-06-9
- Min. Order:
- 10g
- Purity:
- 99.9
- Supply Ability:
- 20kgs