Dabrafenib Mesylate(GSK-2118436B): Pharmacodynamics and Applications in Cancer
General Description
Dabrafenib Mesylate(GSK-2118436B) functions as a kinase inhibitor targeting the BRAF V600E mutation in cancer, working synergistically with trametinib to inhibit the RAS/RAF/MEK/ERK pathway. Clinical studies, like the Phase III COMBI-AD trial, have demonstrated its efficacy in melanoma treatment post-surgery. In anaplastic thyroid cancer, the combination therapy has shown significant clinical activity. Dabrafenib Mesylate(GSK-2118436B) has received approval for various cancer applications including anaplastic thyroid cancer, low-grade glioma in children, melanoma, and non-small cell lung cancer. Ongoing research explores its potential in additional cancer types. Overall, its diverse applications highlight its versatility and promise in improving cancer treatment outcomes.

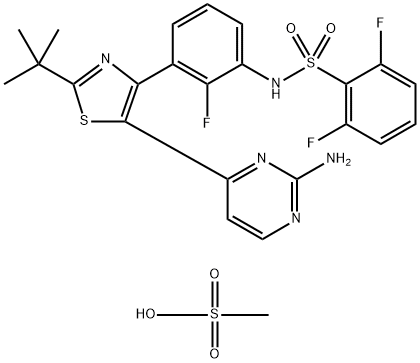
Figure 1. Dabrafenib Mesylate(GSK-2118436B)
Pharmacodynamics
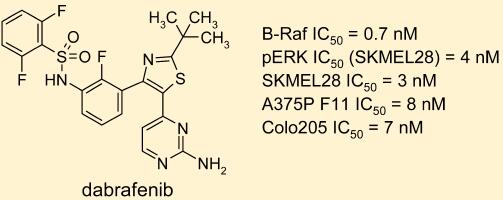
Dabrafenib Mesylate(GSK-2118436B) exerts its pharmacodynamic effects as a kinase inhibitor, primarily targeting the BRAF V600E mutation in various cancer types. By inhibiting the RAS/RAF/MEK/ERK pathway, Dabrafenib Mesylate(GSK-2118436B) works synergistically with trametinib to enhance response rates and reduce resistance without cumulative toxicity. This dual inhibition targets different effectors within the pathway, leading to improved therapeutic outcomes. In melanoma treatment, the efficacy of Dabrafenib Mesylate(GSK-2118436B) in combination with trametinib was demonstrated in the Phase III COMBI-AD study involving 870 patients with Stage III BRAF V600E/K mutation-positive melanoma post-surgery. The primary endpoint of relapse-free survival (RFS) was achieved after a median follow-up of 2.8 years, showcasing the effectiveness of this combination therapy. For anaplastic thyroid cancer, the combination of Dabrafenib Mesylate(GSK-2118436B) and Trametinib has shown significant clinical activity in BRAF V600E-mutated cases, representing a significant advancement in treating this rare disease while maintaining good tolerability. The mechanism of action of Dabrafenib involves competitive and selective inhibition of BRAF by binding to its ATP pocket. While it can inhibit wild-type BRAF, Dabrafenib Mesylate(GSK-2118436B) exhibits higher affinity for mutant forms like BRAF V600E, V600K, and V600D. These mutations lead to constitutive activation of the RAS/RAF/MEK/ERK pathway, crucial for cell cycle regulation, proliferation, and apoptosis inhibition, commonly observed in various cancers. By targeting these specific mutations, Dabrafenib Mesylate(GSK-2118436B) plays a pivotal role in disrupting aberrant signaling pathways and inhibiting tumor growth in affected individuals. 1
Applications in Cancer
Dabrafenib Mesylate(GSK-2118436B) has garnered approval for a range of applications in cancer treatment. Dabrafenib Mesylate(GSK-2118436B) is employed either alone or alongside trametinib dimethyl sulfoxide to target patients exhibiting a specific mutation in the BRAF gene. The approved applications span across various cancer types. Firstly, in the case of anaplastic thyroid cancer, the combination of dabrafenib mesylate and trametinib dimethyl sulfoxide is utilized for cases of locally advanced or metastatic anaplastic thyroid cancer that cannot be managed with local therapy. Additionally, for children aged 1 year and older with low-grade glioma necessitating systemic therapy, Dabrafenib Mesylate(GSK-2118436B), along with trametinib dimethyl sulfoxide, has been approved for use. Furthermore, in the context of melanoma treatment, Dabrafenib Mesylate(GSK-2118436B) is used post-surgery with trametinib dimethyl sulfoxide for patients with lymph node-metastasized melanoma. It can also be employed as a standalone treatment or in combination with trametinib dimethyl sulfoxide for patients with inoperable or metastatic melanoma. Moreover, Dabrafenib Mesylate(GSK-2118436B), combined with trametinib dimethyl sulfoxide, is indicated for metastatic non-small cell lung cancer. Finally, for both adults and children aged 6 years and older, the drug combination is approved for solid tumors that cannot be surgically removed or have metastasized and worsened following other treatments, when other therapies are not suitable. This particular use has been granted approval under the FDA’s Accelerated Approval Program, with the requirement that confirmatory trials must demonstrate clinical benefit. Overall, ongoing research is exploring the potential of Dabrafenib Mesylate(GSK-2118436B) in treating additional types of cancer beyond those currently approved. These diverse applications underscore the versatility and potential of Dabrafenib Mesylate(GSK-2118436B) in addressing various forms of cancer, offering promise for improved treatment outcomes across a broad spectrum of patient needs. 2
Reference
1. Brugnara S, Sicher M, Bonandini EM, Donner D, Chierichetti F, Barbareschi M, Girardelli CR, Caffo O. Treatment with combined dabrafenib and trametinib in BRAF(V600E)-mutated metastatic malignant melanoma: a case of long-term complete response after treatment cessation. Drugs Context. 2018; 15(7): 212515.
2. Dabrafenib Mesylate. NATIONAL CANCER INSTITUTE. 2023.
You may like
Related articles And Qustion
See also
Lastest Price from Dabrafenib Mesylate manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 1195768-06-9
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month
US $0.00/g2025-04-21
- CAS:
- 1195768-06-9
- Min. Order:
- 10g
- Purity:
- 99.9
- Supply Ability:
- 20kgs