Chlorambucil: History and It-Based Hybrid Compounds with Anticancer Activity
Chlorambucil is an immunosuppressant that is commonly prescribed by veterinarians to treat dogs and cats that are suffering from immune-mediated disorders and cancers, such as lymphoma and leukemia. The drug also has antineoplastic properties that make it effective for ridding the pet's body of unhealthy cellular growths in the blood and lymphatic systems.

Pharmacodynamics
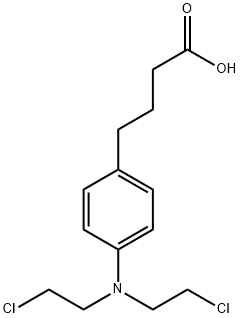
Chlorambucil is an antineoplastic in the class of alkylating agents that is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn inhibits their correct utilization by base pairing and causes a miscoding of DNA. Alkylating agents are cell cycle-nonspecific. Alkylating agents work by three different mechanisms all of which achieve the same end result - disruption of DNA function and cell death.
Chlorambucil-Based Hybrid Compounds with Anticancer Activity
Increasing cases of cancer have been a primary concern in recent decades. Developing new chemotherapeutics is challenging and has been faced with limitations, such as multidrug resistance, poor specificity, selectivity, and toxicity. The aforementioned factors contribute to treatment failure. Hybrid compounds have features that can overcome the limitations mentioned above. Chlorambucil, an anticancer drug that is used to treat prostate and breast cancer, suffers from poor aqueous solubility and specificity, a short half-life, and severe side effects, including anaemia and bone marrow suppression. It compromises the immune system, resulting in treatment failure. Hence, its combination with other pharmacophores has been reported to result in effective anticancer agents with fewer side effects and high therapeutic outcomes.1
Reference
1. Peter S, Aderibigbe BA. Chlorambucil-Bearing Hybrid Molecules in the Development of Potential Anticancer Agents. Molecules. 2023; 8(19): 889.
Related articles And Qustion
See also
Lastest Price from Chlorambucil manufacturers

US $0.00/g/Bag2025-04-21
- CAS:
- 305-03-3
- Min. Order:
- 10g
- Purity:
- 99%min; USP
- Supply Ability:
- 60kg/month

US $0.00-0.00/Kg2020-02-26
- CAS:
- 305-03-3
- Min. Order:
- 1KG
- Purity:
- 99.0%+
- Supply Ability:
- 1000 tons