The reactions of Zinc ammonium chloride
Zinc ammonium chloride is an inorganic compound with the formula (NH4)2ZnCl4. It is the ammonium salt of tetrachlorozincate. It is used as a flux in the process of hot-dip galvanizing.
The zinc ammonium chloride reagents can reduce a wide range of functional groups. Reducing azides to amines is of considerable importance for introducing primary amino groups in organic synthesis because of the easy preparation of azides by regio- and stereocontrolled procedures. Zinc and ammonium chloride reduce a broad spectrum of alkyl or acyl azides to amines or amides under mild conditions. Zinc ammonium chloride has affected the conversion of nitrophenols into amino phenols, not to the respective hydroxylamine. A variety of nitroalcohols can be reduced in a chemoselecive manner with aqueous zinc ammonium chloride and (Boc)2O to the corresponding b-hydroxycarbamates. Other functional groups, such as carbonyl, halide, and also double bonds, remain unaffected under these reaction conditions.
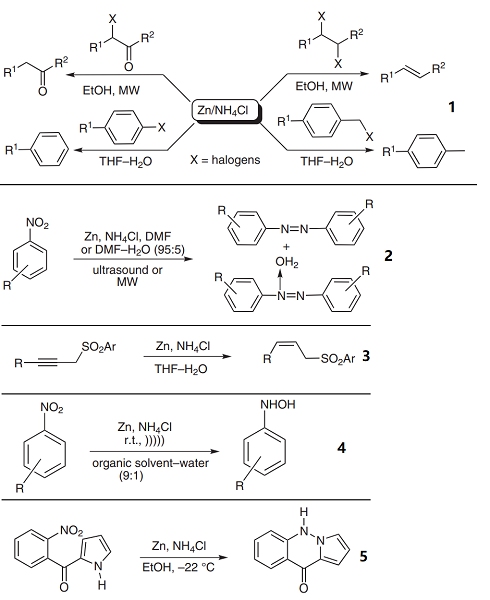
1. The reductive elimination of halogens from aromatic rings and aliphatic chains is quite a difficult task. The low-cost and highly effective reagent, zinc ammonium chloride, has been used for the dehalogenation of aryl halides and alkyl halides in different solvent systems.
2. The chemoselective reduction of nitroarenes to azo or azoxy compounds can be easily achieved by means of zinc powder and ammonium chloride.
3. Sheldrake et al. have revealed that propargylic sulfones can be cis-hydrogenated using commercial zinc powder and ammonium chloride with good chemoselectivity. Other reducible groups, such as alkene and benzyloxy, are not affected.
4. Reduction by zinc in the presence of ammonium chloride in an aqueous solution is one of the methods available for preparing hydroxylamines from the corresponding nitro compounds.
5. The reduction of 2- and 3-acylpyrroles can be carried out with zinc and ammonium chloride in ethanol at low temperatures to produce the corresponding fused heteroaromatic system.
6. b-Keto acyloxy amides are readily transformed into the corresponding b-keto amides by reductive deacetylation in extraordinarily mild conditions, using activated zinc metal in a mixture of saturated aqueous ammonium chloride and methanol.
7. Habashita et al. described a convenient and efficient method for the synthesis of synthetically useful non-racemic allylic alcohols from 4-methylbenzenesulfonates of non-racemic 2,3-epoxy alcohols by reaction with potassium iodide followed by zinc powder and ammonium chloride in a one-pot manner.
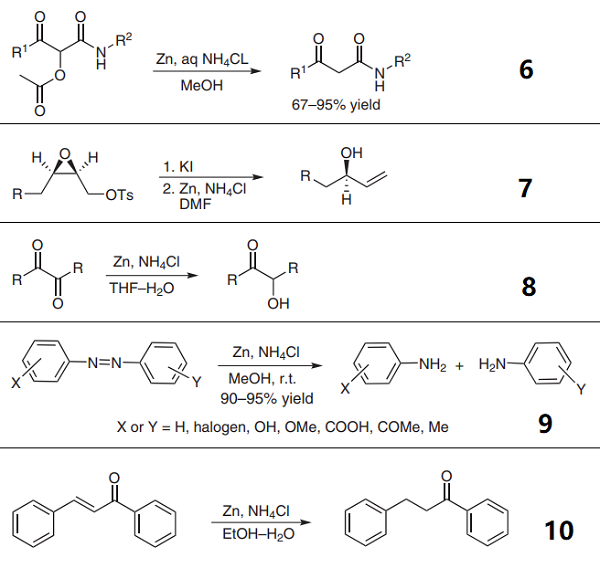
8. Problems arise from the overproduction of diketones to diols or to monotones. Hekmatshoar et al. successfully employed zinc in a THF-saturated aqueous ammonium chloride reduction system to convert 1, 2-diketones into a-hydroxy ketones.
9. Azo compounds can be productively reduced to the corresponding anilines with zinc dust in the presence of ammonium chloride.
10. The chemoselective reduction of conjugated carbonyl compounds is a useful functional group transformation in organic synthesis. Zinc ammonium chloride was applied for the selective 1,4-reduction of chalcones under mild conditions with high selectivity.
Reference
[1] Saikia, Bishwajit. “Zinc Ammonium Chloride.” Synlett 24 1 (2011): 2597–2598.
You may like
Lastest Price from Zinc ammonium chloride manufacturers

US $6.00/kg2025-04-21
- CAS:
- 14639-97-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/KG2025-04-15
- CAS:
- 14639-97-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg