Synthesis of Ferulic acid
General description
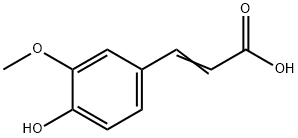
Ferulic acid is a ferulic acid consisting of trans-cinnamic acid bearing methoxy and hydroxy substituents at positions 3 and 4 respectively on the phenyl ring. It has a role as an antioxidant, a MALDI matrix material, a plant metabolite, an anti-inflammatory agent, an apoptosis inhibitor and a cardioprotective agent. It is a conjugate acid of a ferulate.[1]
Ferulic acid ([E]-3-[4-hydroxy-3-methoxy-phenyl] prop-2-enoic acid) (Fig. 1) belongs to the phenolic acid group commonly found in plant tissues [1]. Phenolic acids are secondary metabolites of varying chemical structures and biological properties. The plants are mainly found in bound form as ester or glycosides, lignin components, and hydrolysis tannins. In terms of chemical structure, they can be divided into derivatives of cinnamic and benzoic acid, varying in number and substitution of hydroxyl and methoxy groups, and phenolic acidsof unusual character. An additional group is the depside,which is a combination of two or more phenolic acids. Ferulic acid, like caffeic, p-coumaric, synapine, syryte, and vanillin acids, is the most common cinnamic acid derivative.
Application and pharmacology
As a derivative of ferulic acid,ferulic acid ester has various biological activities,such as anti-thrombosis,anti-nflammatory,analgesic,and regulation of human immune function,which is widely used in medicine,food,cosmetics and so on.Ferulate has a variety of health functions, such as protecting liver, protecting intestines and stomach, enhancing immunity, increasing food flavor and so on. Therefore, as a health food and food additive, it will have a market.
Ferulic acid has low toxicity and possesses many physiological functions (anti-inflammatory, antioxidant, antimicrobial activity, anticancer, and antidiabetic effect). It has been widely used in the pharmaceutical, food, and cosmetics industry. Ferulic acid is a free radical scavenger, but also an inhibitor of enzymes that catalyze free radical generation and an enhancer of scavenger enzyme activity. Ferulic acid has a protective role for the main skin structures: keratinocytes, fibroblasts, collagen, elastin. It inhibits melanogenesis, enhances angiogenesis, and accelerates wound healing. It is widely applied in skin care formulations as a photoprotective agent, delayer of skin photoaging processes, and brightening component. Nonetheless, its use is limited by its tendency to be rapidly oxidized.[1]
The regulation of brown rice ferulate on blood lipid. After feeding rats with hyperlipidemia for 6 weeks, they found that the blood lipid content of rats decreased significantly, the spleen gland index increased significantly, the fat content in liver decreased significantly, and the inhibition rates were higher than 30%, 45% and 30% respectively, It shows that brown rice ferulate has a good preventive effect on fatty liver. Others found that ferulate can reduce the conversion of alcohol to acetaldehyde, reduce the content of triacylglycerol in the liver group, reduce the activities of alanine aminotransferase and aspartate aminotransferase in the body, and improve the levels of acetaldehyde dehydrogenase, alcohol dehydrogenase and glutathione, It effectively reduces the damage of alcohol to the liver.
1.Ferulic acid is widely applied in skin care formulations as a delayer of skin photoaging processes and photoprotective agent. Its application as a topical antioxidant has become an important administration route due to maintaining a high local concentration and the low cutaneous metabolism
2. Ferulic acid is used in the production of face masks, as well as antioxidant, protective, and moisturizing creams/lotions. The recommended acid concentration in cosmetic products of this type is from 0.5 to 1%. Ferulic acid is also used in medical cosmetology and aesthetics salons. It is most often used at a concentration of 12% and in combination with vitamins C and hyaluronic acid.
Synthesis
Generally speaking, ferulic acid can be esterified with most alcohols to produce ferulate derivatives. However, the reaction process is reversible, there are many impurity products after the reaction, and the purity of ferulate is small, so its yield is low. Others studied the esterification of ferulic acid and ethanol using concentrated hydrochloric acid as catalyst. The results show that the yield of ethyl ferulate is low, there are many impurities, and a large amount of wastewater will be produced, which will do some harm to the environment. A dry closed container, filled with nitrogen as the protective gas, and then added ferulic acid and ethanol. The results showed that this method could effectively promote the production of ferulate. The results showed that the yield of ferulic acid ester was significantly increased, and the whole process was easy to operate, without too much wastewater and impurities. However, the cost in this process is high, which is not conducive to the actual production of ferulate. Describe the synthesis method of ferulic acid ester,including acid alcohol esterification reaction,acid chloride alcoholization reaction,transesterification reaction,enzyme catalyzed reaction[2].
Figure 1. the systhesis route of FA
Song Fanfan, sun Shangde[3] (figure.1) and others reacted ethyl carotene with ethyl ferulate, and then added 2-ethylhexanol for transesterification. As a result, the content of ferulate was close to 58%. Others used ferulic acid, triglyceride and triglyceride to carry out transesterification reaction. After 7 days of reaction, ferulic acid triglyceride and triglyceride were obtained, and the yields were 8.6% and 9.2% respectively.
Source
Ferulic acid is most commonly found in whole grains, spinach, parsley, grapes, rhubarb, and cereal seeds, mainly wheat, oats, rye, and barley (Table 1). One of the most important role of phenolic acids, especially cinnamic acid derivatives, is their antioxidant activity, which depends primarily on the number of hydroxyl and methoxy groups attached to the phenyl ring [3, 4]. Ferulic acid is more easily absorbed into the body and stays in the blood longer than any other phenolic acids. Ferulic acid is considered to be a superior antioxidant.
Toxicity
Research conducted so far has shown that ferulic acid has strong antioxidant properties, which is directly involved with its protective role to cellular structures and inhibition of melanogenesis. It is increasingly used in cosmetic preparations, mainly to inhibit photostage. At the same time, it helps to reduce fine wrinkles and existing discoloration. Good penetration into the skin, compatibility with many cosmetic formulas, and stabilizing properties of other ingredients make ferulic acid an increasingly used compound in cosmetology.
Storage
Closed light sealed packaging. Store in cool and dry warehouse. Keep away from heat source and do not store together with toxic chemicals.
Reference
1.Zduńska K., Dana A. & Kolodziejczak A. et al., "Antioxidant Properties of Ferulic Acid and Its Possible Application," Skin Pharmacology and Physiology, Vol.31, No.6(2018), pp.332-336.
2.Song Fanfan,Progress in synthesis of fat soluble ferulic acid derivatives_ .
3.Li Xiugang, Zhang Lingyu: Research Progress on Synthesis and application of ferulic acid, Shandong chemical industry, 2020, No. 17, pp. 81-82.
You may like
Related articles And Qustion
Lastest Price from Ferulic Acid manufacturers

US $1200.00-1100.00/ton2025-11-10
- CAS:
- 1135-24-6
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00/kg2025-08-17
- CAS:
- 1135-24-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 50000