Synthesis of Bremelanotide
Background
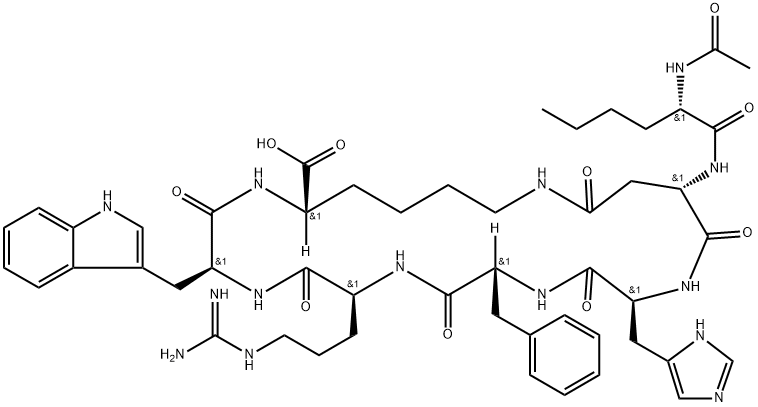
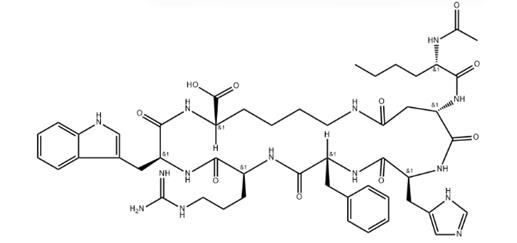
Bremelanotide (Bremelanotide), is that a kind of α-melanophore stimulates hormone (α-melanocyte stimulating hormone, α MSH) polypeptide alpha analog also known as Pu Meila peptides, PT141, is ring seven peptide structure.Numerous studies have proven to their acceptor molecule and play an important role in sexual arousal, in reducing appetite and the melanic synthesis of catalysis, it is possible thereby to the treatment of sexual dysfunction, fat and stimulation skin produce the diseases such as Melanocortins opposing ultraviolet burn.PT-141 It is a kind of medicine of the treatment of sexual dysfunction of Palatin companies of U.S. exploitation, it is clinical to have completed the III phases at present, all effective to men and women patient, especially the only effective drug candidate of female frigidity patient.
Existing Bremelanotide synthetic method is based on solid phase synthesis; Chinese patent 200710048824.6 discloses a kind of solid phase synthesis process using Fmoc (fluorenylmethyloxycarbonyl) protections; Pu Meila peptide of the solid phase synthesis with protection group first; secondly Pu Meila peptides are cyclized on resin; then purification is cut from resin; acetic acid Pu Meila peptides are then converted to, yield is 17%.Chinese patent 200610086841.4 discloses the solid phase synthesis process of PT-141; the invention removes partial side-chain protection group by selectively protecting partial amino-acid after synthesis heptapeptide, is cyclized using different cyclization orders; cracking obtains after purification purpose product, and yield is less than 40%.The above-mentioned synthesis main method for Bremelanotide, has that synthetic route is long, and step is more; synthetic route is single, and time-consuming, high cost; the protective group base of Individual amino acids is interfered in the problems such as yield is not high, and building-up process, significantly increases the reaction selectivity of protective group base.
Chemical Synthesis
1. HMBA-Rink-Amide-AM-resin resins are prepared
Rink-Amide-AM-resin resins are taken out, glass polypeptides reactive post the inside is put into, be washed once with DMF, then with DCM swelling 20 minutes or so, pump DCM;HMBA linker, HOBt, DIC of the 4 times of amounts dissolved with DMF, 2 hours of coupled reaction, triumphant plucked instrument reagent detection is added if colourless, to represent that reaction is complete, if also color, then it represents that complete without reaction, repeat coupling process, till reaction is completely.Then washed with DMF four times.
2. Fmoc-Lys (IVDde)-HMBA-Rink-Amide-AM-resin resins are prepared
Fmoc-Lys (IVDde)-OH, HOBt, DMAP, the DIC mixed with DMF is added inside the glass polypeptides reactive post of step 1, they are with the mol ratio of resin(3/3/0.3/3.6):1, more than 5 hours of reaction, then washed four times with DMF, add the 20-30 times of acetic anhydride measured and pyridine mixed liquor(Ratio is 2:1), overnight, or more than 10 hours of closing, with DMF, DCM is alternately washed 6 times, uses methanol shrinkage resin, five minutes every time, vacuum dry adsorbent for closing.
3. The measurement of Fmoc-Lys (IVDde)-HMBA-Rink-Amide-AM-resin substitution degrees
The resin obtained in 5-10mg steps 2 is taken, in being put into the EP pipes of 1.5mL, the DBLK of 1mL is added, 20-30min is shaken.Standing takes supernatant, prepares the little volumetric bottle of 2 10mL, and a DBLK that 100ul is added as blank the inside, the supernatant of another addition 100ul is separately added into DMF and quantitatively arrives 10mL, shakes up.With ultraviolet spectrophotometer wavelength be at 290nm detect, reading numerical values.According to formula, calculating substitution degree is0.3mmol/g.Formula is:51* measure numerical value/(5.8* weighs amount of resin).
4. The synthesis of Fmoc-Trp (boc)-Lys (IVDde)-HMBA-Rink-Amide-AM-resin
According to the scale to be synthesized, the resin prepared in respective quality step 2 is weighed(The quality * resin substitution degree of the molal quantity=resin of synthesis)3.3g, i.e. 0.99mmol are put into glass polypeptides reactive post the inside, washed once with DMF, then with DCM swelling 20 minutes or so, pump DCM, add DBLK, carry out deprotection(Slough interim protection group Fmoc), being removed twice with DBLK respectively, the time is 10+15 minutes, and centre washed once with DMF.After second deprotection terminates, with DMF, DCM alternately washing resin five times respectively; add Fmoc-Trp (boc)-OH, the mixing DMF solution of HOBt, DIC of 4 times of amounts; after coupled reaction 2 hours, triumphant plucked instrument reagent detection, if colourless; represent that reaction is complete; if also color, then it represents that complete without reaction, the response time should be extended; or repetition above method carries out repeating coupling, till seal is colourless.Then washed with DMF three times.
5. The synthesis of Fmoc-Arg (pbf)-Trp (boc)-Lys (IVDde)-HMBA-Rink-Amide-AM-resin
After step 4, enter DBLK, carry out deprotection(Slough interim protection group Fmoc), being removed twice with DBLK respectively, the time is 10+15 minutes, and centre washed once with DMF.After second deprotection terminates, with DMF, DCM alternately washing resin five times respectively; add Fmoc-Arg (the pbf)-OH, HOBt, DIC of 4 times of amounts; mixing DMF solution, after reaction 2 hours, the detection of triumphant plucked instrument reagent; promise is colourless, represents that reaction is complete, if also color; then represent complete without reaction; the response time should be extended, or repeat above method carries out repeating coupling, till seal is colourless.Then washed with DMF three times.
6. The synthesis of Fmoc-D-Phe-Arg (pbf)-Trp (boc)-Lys (IVDde)-HMBA-Rink-Amide-AM-resin
After step 5, enter DBLK, carry out deprotection(Slough interim protection group Fmoc), being removed twice with DBLK respectively, the time is 10+15 minutes, and centre washed once with DMF.After second deprotection terminates, with DMF, DCM alternately washing resin five times respectively; add the Fmoc-D-Phe-OH of 4 times of amounts, the mixing DMF solution of HOBt, DIC; after reaction 2 hours, triumphant plucked instrument reagent detection, promise is colourless; represent that reaction is complete; if also color, then it represents that complete without reaction, the response time should be extended; or repetition above method carries out repeating coupling, till seal is colourless.Then washed with DMF three times.
7. The synthesis of Boc-His (trt)-D-Phe-Arg (pbf)-Trp (boc)-Lys (IVDde)-HMBA-Rink-Amide- AM-resin
After step 6, enter DBLK, carry out deprotection(Slough interim protection group Fmoc), being removed twice with DBLK respectively, the time is 10+15 minutes, and centre washed once with DMF.After second deprotection terminates, with DMF, DCM alternately washing resin five times respectively; add Boc-His (the trt)-OH, HOBt, DIC of 4 times of amounts; mixing DMF solution, after reaction 2 hours, the detection of triumphant plucked instrument reagent; promise is colourless, represents that reaction is complete, if also color; then represent complete without reaction; the response time should be extended, or repeat above method carries out repeating coupling, till seal is colourless.Then washed with DMF three times.
8. The removing of Side chain protective group IVDde in Boc-His (trt)-D-Phe-Arg (pbf)-Trp (boc)-Lys (IVDde)-HMBA-Rink-Amide- AM-resin resins
After step 7, with 5% hydrazine hydrate, resin is removed twice, 20 minutes or so every time, then with DMF, DCM replaces respectively washing resin five times.
9. Synthesis
After step 8, AC-Nle-Asp (the COOH)-otbu, HOBT of 4 times of amounts is added, the mixing DMF solution of DIC, after reaction 2 hours, triumphant plucked instrument reagent detection, promise is colourless, represent that reaction is complete, if also color, then it represents that complete without reaction, the response time should be extended, or repetition above method carries out repeating coupling, till seal is colourless.Then DMF, DCM alternately washing 6 times, then use methanol shrinkage resin, five minutes every time, vacuum dry adsorbent.
10 cracking
After resin is dried, weigh, by 1:10 resin and the ratio of lysate.Resin is cracked, the proportioning of lysate is(TFA:Thioanisole:Methyl phenyl ethers anisole:EDT=90:5:2:3), 2 hours of pyrolysis time or so.After cracking terminates, filtered with sand core funnel, leave filtrate, filtrate is settled in methyl tert-butyl ether, be then centrifuged for, be vacuum dried, obtain thick peptide。( 0.99mmol The resin of amount obtains thick peptide 1.14g Left and right, yield exists 98% Left and right( 1mmol Amount, theoretical yield 1.174g ))
11. oxidative synthesis
Thick peptide is dissolved in into DMF/DCM=2.5:In 1 mixed solvent, dissolve according to the ratio of 6mmol/L, addition PyBop.HOBt.DIPEA. (and the mol ratio of thick peptide is respectively:1:3:3:3), be cyclized 2-4 hour, during can detect with HPLC, confirm to react completely.Just obtain
12. hydrolysis are obtained
Above-mentioned solution is rotated, the DCM of the inside is evaporated, then in the ultra-pure water of the three times for adding remaining liq volume, be subsequently adding dilute NaOH solution, adjust PH=10-11, stir half an hour, then complete hydrolysis fall HMBA.
13. acid addings are neutralized
The fine work of 14. Bremelanotides
15. efficient liquid phase preparation methoies
High performance preparative liquid chromatography instrument uses front preparation:A phases prepare aqueous solution for above-mentioned, and B phases prepare acetonitrile solution for above-mentioned, and the DAC for selecting C18 diameter 50MM prepares post, and flow velocity is 40ML/MIN.Column temperature is 25 degree, wavelength 220nm, and thick peptide 500ml pumps into liquid phase and is prepared after above-mentioned cyclisation is filtered, and preparation gradient is time 0-7-50 (MIN), and concentration is 5-29-39(B%), appearance time is 25-30MIN, and appearance is B% concentration when being 35%.Receiving peak height is>150MV is Brehme Lang Dan sterlings.Finally obtain purity 99.2% Sterling above 0.587 Gram or so, the Brehme Lang Dan response rate 57.3% 。
You may like
Related articles And Qustion
Lastest Price from BREMELANOTIDE manufacturers

US $10.00/box2025-12-08
- CAS:
- 189691-06-3
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 9999

US $5.00-0.50/Gram2025-11-21
- CAS:
- 189691-06-3
- Min. Order:
- 1Gram
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS