Introduction of Aluminum chloride
General description
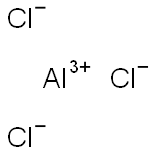
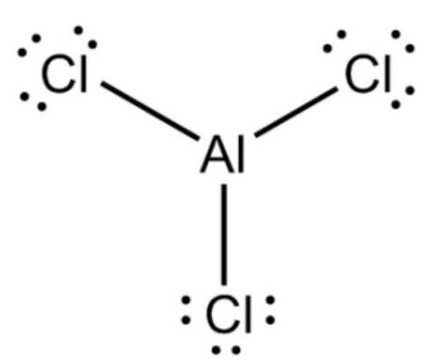
Aluminum chloride is an inorganic substance with the chemical formula AlCl3. It is a compound of chlorine and aluminum. Aluminum chloride has low melting point and boiling point and sublimates into covalent compounds. Molten aluminum chloride is not easy to conduct electricity, which is different from most salts containing halogen ions (such as sodium chloride). AlCl3 is a "YCl3" structure, which is the most densely stacked layered structure of Al3 + cube, while Al3 + in AlBr3 accounts for the adjacent tetrahedral gap of BR − most densely stacked frame. During melting, AlCl3 generates volatile dimer al2cl6, which contains two three center four electron chlorine bridge bonds. At higher temperature, al2cl6 dimer dissociates to form planar triangular AlCl3, which is similar to boron trifluoride (BF3). Aluminum chloride is a white crystalline powder. When aluminum chloride vapor is dissolved in non-polar solvents or in molten state, it exists in the form of covalent dimer molecule (al2cl6). It can be dissolved in water and many organic solvents. The aqueous solution is acidic. In the presence of aromatics, aluminum chloride.[1]
Application and pharmacology
Aluminum chloride is a chemical compound with the chemical formula AlCl3. When contaminated with iron chloride, it often displays a yellow color compared to the white pure compound. It is used in various chemical applications as a Lewis base, with anhydrous aluminium trichloride being the most commonly used Lewis acid. It may also be found in over-the-counter as an antiperspirant or prescription products as an antihemorrhagic agent. In antiperspirant products, FDA approves the use of aluminum chloride as an active ingredient up to 15%, calculated on the hexahydrate form, in an aqueous solution nonaerosol dosage form.
Treatment of plantar hyperhidrosis has used various strategies. The first line of treatment has been application of an antiperspirant, such as topical aluminum chloride. In2011, Streker and colleagues1conducted a study on the effectiveness of two different concentrations of aluminum chloride, 12.5% and 30%, and found that both significantly reduced sweat production in plantar hyperhidrosis in 6 weeks. Because both were effective, they recommend using aluminum chloride 12.5%.1Although skin irritation is possible with this treatment, the authors applied the aluminum chloride with a roller and had minimal occurrence of irritant dermatitis, which did not interfere with treatment.[2]
Physical Properties
Aluminum chloride, white crystalline powder, strong smell of hydrochloric acid, industrial products are light yellow. Soluble in water, alcohol, chloroform, carbon tetrachloride, slightly soluble in benzene. Molten aluminum chloride is not easy to conduct electricity, which is different from most salts containing halogen ions (such as sodium chloride). The aqueous solution of aluminum chloride is completely dissociated and is a good conductor. Aluminum chloride sublimates at 178 ℃, and its vapor is associated bimolecular. It can absorb water in the air and partially hydrolyze to release hydrogen chloride. Aluminum chloride is colorless transparent crystal or white and slightly yellowish crystalline powder. It is easy to absorb water and partially hydrolyze to release hydrogen chloride to form acid mist. Soluble in water and strongly hydrolyzed, the solution is acidic. It is also soluble in ethanol and ether and emits a lot of heat at the same time.
Synthesis
Laboratory preparation method aluminum and hydrochloric acid are prepared at room temperature (2Al+6HCL= 2AlCl3+3H2↑). The preparation can be accelerated under heating conditions. If anhydrous aluminum chloride is prepared from crystalline aluminum chloride, because it is very easy to hydrolyze, it needs to be heated in hydrogen chloride atmosphere to lose crystalline water, or treated with sulfite chloride and other dehydrating agents.
Industrial preparation method:
1.It can also be prepared by the reaction of alumina and carbon tetrachloride at room temperature (Al2O3 + 3CCl4 = 2AlCl3 + 3COCl2)
2. Aluminum oxide powder method: put the industrial alumina with a certain particle size and petroleum coke into the roasting furnace in a certain proportion for mixing Evenly, air is introduced from the furnace bottom for roasting. The roasted material enters the chlorination furnace, chlorine and oxygen are introduced into the furnace, and aluminum oxide powder reacts with chlorine in the presence of reducing agent carbon. The generated gas phase product is pre cooled, purified and removed to the trap to prepare aluminum chloride finished product. The tail gas is drained after being absorbed by sodium hydroxide or sodium sulfite solution.
Toxicity
Aluminum chloride, solution appears as a straw-colored liquid. Denser than water. Contact may cause severe irritation to skin, eyes, and mucous membranes. May be toxic by ingestion. Aluminum chloride, anhydrous appears as a white to gray powder with a pungent odor. Corrosive to tissue and toxic by ingestion. Inhalation of high concentration aluminum chloride can stimulate the upper respiratory tract to produce bronchitis, and stimulate the skin and mucous membrane. Individuals can cause bronchial asthma. When taken by mistake, it can cause oral erosion, gastritis, gastric bleeding and mucosal necrosis.Chronic effects: long term exposure can cause headache, dizziness, anorexia, cough, nasal congestion, chest pain and other symptoms. Acute toxicity: ID503730mg/ kg (oral to rats); hazard characteristics: react with water and give off toxic corrosive gas; combustion (decomposition) products: chloride and alumina.[3]
Reference
1.Balmaev B. G., Vetchinkina T. N. & Lysenko A. P. et al., "Prospects of Aluminum Chloride Electrolysis under Modern Conditions," Russian metallurgy Metally, Vol.2021, No.6(2021), pp.667-671.
2.Vlahovic T. C., "Plantar Hyperhidrosis," Clinics in Podiatric Medicine and Surgery, Vol.33, No.3(2016), pp.441-451.
3.Bergfors E., Inerot A. & Falk L. et al., "Patch testing children with aluminium chloride hexahydrate in petrolatum: A review and a recommendation," Contact Dermatitis, Vol.81, No.2(2019), pp.81-88.
You may like
Related articles And Qustion
Lastest Price from Aluminum chloride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 7446-70-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $1.10/g2025-04-17
- CAS:
- 7446-70-0
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min