Introduction, biological activity and clinical application of axitinib.
General description
Axitinib is a potent and selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3 that is approved in the US and several other countries for treatment of patients with advanced renal cell carcinoma after failure of one prior systemic therapy [1]. The recommended clinical starting dose of axitinib is 5 mg twice daily, taken with or without food. Dose increase (up to a maximum of 10 mg twice daily) or reduction is permitted based on individual tolerability. Axitinib pharmacokinetics are dose-proportional within 1–20 mg twice daily, which includes the clinical dose range. Axitinib has a short effective plasma half-life (range 2.5–6.1 h), and the plasma accumulation of axitinib is in agreement with what is expected based on the plasma half-life of the drug [2-4].
Axitinib is an oral inhibitor of the VEGF, PDGF and colony stimulating factor-1 receptor tyrosine kinases and is currently in development by Pfizer Inc for the potential treatment of various solid tumors [5]. Phase II trials with this agent alone or in combination with chemotherapeutic drugs were reported in several types of malignancy, with activity observed in thyroid, pancreatic, lung, renal, breast and colorectal cancers, melanoma and other carcinomas. Although frequent side effects have included fatigue, hypertension, diarrhea, hand-foot syndrome and proteinuria, axitinib was well tolerated overall. Larger, randomized phase II/III studies were ongoing at the time of publication [6].
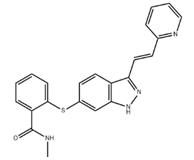
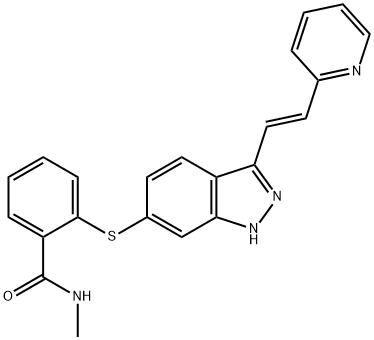
Fig. 1 The structure of axitinib.
Bioactivities
(1) Axitinib Anti-VEGFR Activity
Axitinib, a small molecule indazole derivative, is an oral, potent, and highly selective inhibitor of VEGFR-1, -2, and -3. The structure-based drug design of axitinib allows strategic optimization of critical binding elements, with the tight fit of axitinib into the ‘deep pocket’ confirmation of the kinase domain of VEGFRs resulting in high potency and selectivity [7]. In vitro, axitinib inhibits VEGFR-1, -2, and -3 autophosphorylation at picomolar concentrations, consistent with its potent and highly selective activity against these receptors. Indeed, the half maximal inhibitory concentration (IC50) for axitinib is 10-fold lower for the VEGF family receptors than for RTKs of other family receptors. The IC50 for axitinib is also lower than other RTK inhibitors for most of the targets of interest. The relative potencies of axitinib and other agents targeting VEGFRs -1, -2, and -3 in mRCC are illustrated. Additionally, studies carried out in vitro showed that axitinib did not significantly inhibit other receptor kinases that were tested, including colony-stimulating factor (CSF)-1R, fms-like tyrosine kinase (Flt)-3, fibroblast growth factor receptor (FGFR)-1, ret proto-oncogene (RET), epidermal growth factor receptor (EGFR), and met proto-oncogene encoding hepatocyte growth factor (c-Met) [8-10].
(2) Axitinib Antitumor Activity
Axitinib has demonstrated in vivo target modulation and antiangiogenesis in several nonclinical models. In xenograft tumor mouse models across various tumor types, axitinib antitumor activity was associated with marked reduction in tumor vascularization and blood flow, and tumor shrinkage [11]. These were achieved at a range of plasma concentrations consistent with its in vitro potency and were dose dependent. The Ctarget value (required in vivo pharmacologic concentration) was determined to be ∼0.5 nmol/L (unbound), which in humans would be equivalent to a Ctarget of ∼100 nmol/L (total) [12].
In a phase I study of axitinib in patients with advanced solid tumors, the maximum tolerated dose and recommended dose in humans was determined to be 5 mg twice daily on a continuous daily dosing schedule. Hypertension and stomatitis were the main dose-limiting toxicities seen during the phase I study; these were mainly observed with higher doses of axitinib. Other adverse events (AEs) included fatigue, diarrhea, nausea, and vomiting [13].
Pharmacokinetics
Axitinib is administered orally on a proposed standard dosing regimen of 5 mg twice daily with titration as required, and is rapidly absorbed, with peak plasma concentrations measurable within 2–6 hours after dosing in the fed state. Axitinib exhibits linear pharmacokinetics at doses of 2–10 mg twice daily. Peak plasma concentrations are reached in 1–2 hours after dosing in the fasted state, while plasma half-life remains unchanged. Axitinib exists in different crystal forms [14-15]. With crystal polymorph Form IV, higher plasma levels were observed after overnight fasting, but not with shorter fasting times (e.g. fasting 1 or 2 hours before and after each dose). Findings for polymorph Form XLI are awaited.
Axitinib primarily undergoes hepatic metabolism via the cytochrome P450 (CYP) 3A4 isozyme, with some additional metabolism occurring via oxidation by CYP2C19 and CYP1A2 and glucuronidation via uridine diphosphate glucuronosyltransferase (UGT) 1A1. The major circulating axitinib metabolites, the glucuronide and the sulfoxide, are not active. Less than 1% of the administered drug appears as unchanged drug in the urine [16]. No changes in the pharmacokinetic profile of axitinib or comparator agents were observed when axitinib was administered in combination with chemotherapies including paclitaxel, docetaxel, cisplatin, carboplatin, oxaliplatin, 5-fluorouracil, irinotecan, and/or gemcitabine [17].
Pharmacodynamics
In a study measuring the exposure-response relationship of axitinib in patients with advanced solid tumors, a >50% decrease in the volume transfer coefficient (Ktrans) and initial area under the curve was demonstrated by day 2 of therapy, and persisted through week 4 of treatment (figure 3). A linear correlation suggested that increased axitinib exposure is associated with higher efficacy, as indicated by decreased tumor perfusion and volume [18].
Safety and tolerability
For single-agent axitinib, the most common AEs reported are hypertension, fatigue, and gastrointestinal toxicity. These AEs are an expected class effect due to the known mechanism of action of the drug [19]. The majority of AEs are manageable with dose modification and supportive care; hypertension is generally easily managed with standard antihypertensive drugs.
In sorafenib-refractory patients, the most common all-causality AEs were fatigue (77%), diarrhea (61%), anorexia (48%), and hypertension (45%). All-causality grade 3–4 AEs included hand-foot syndrome (HFS; 16.1%), fatigue (16.1%), hypertension (16.1%), dyspnea (14.5%), diarrhea (14.5%), dehydration (8.1%), and hypotension (6.5%). Of 62 patients enrolled, treatment was discontinued in 22 patients due to AEs (of these, 12 were attributed to study treatment) [20].
Common treatment-related AEs reported in the Japanese trial of cytokine-refractory patients were HFS (73%), hypertension (66%), diarrhea (66%), and hoarseness (53%). In all, 18 patients developed proteinuria ≥2 g/24 hours during treatment and required dose reduction or treatment interruption/discontinuation [21]. Of note, the incidences of HFS and proteinuria in the Japanese study were higher than those reported in the Western study in cytokine-refractory mRCC patients.
Application
Axitinib is an oral TKI that has been mainly developed for the treatment of RCC. Its advantages over other TKIs are a potent VEGFR inhibition, little off VEGFR-targeted effect, and an individualized prescription based on clinical dose titration. These advantages led to the demonstration of a clinical benefit compared to standard dose sorafenib and approval of axitinib as second-line treatment in RCC patients, but axitinib failed to demonstrate its superior efficacy as first line over other TKIs. Recently, axitinib have been replaced with cabozantinib and nivolumab in the second-line setting in international guidelines and its place in therapy therefore remains to determine[22]. Trials are currently ongoing to assess its efficacy in the adjuvant setting or in the first-line setting in combination with immunotherapies. additionally axitinib confirmed its dose-dependent inhibition on angiogenesis and tumor growth in mice, including in xenograft model of human tumor: axitinib caused regression of the tumor vasculature, with loss of endothelial sprouts and fenestration and decreased vessels density[23-24].
References
[1] B.I. Rini, J.J. Moslehi, M. Bonaca, M. Schmidinger, L. Albiges, T.K. Choueiri, R.J. Motzer, M.B. Atkins, J. Haanen, M. Mariani, J. Wang, S. Hariharan, J. Larkin, Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients With Advanced Renal Cell Cancer: Data From the Phase III JAVELIN Renal 101 Trial, J. Clin. Oncol. 40(17) (2022) 1929-1938.
[2] K. Li, Y. Li, Y. Lyu, L. Tan, X. Zheng, H. Jiang, H. Wen, C. Feng, Development of a phagocytosisdependent gene signature to predict prognosis and response to checkpoint inhibition in clear-cell renal cell carcinoma, Front. Immunol. 13 (2022) 853088.
[3] M. Amin, T.L.M. Ten Hagen, Auristatin-loaded liposomes and uses thereof, Erasmus University Medical Center Rotterdam, Neth. . 2022, p. 91pp.
[4] X. Hang, L. Zhao, B. Wu, S. Li, P. Liu, J. Xu, X. Wang, P. Chi, C. Chen, T. Niu, L. Dai, Y. Liu, BCL-2 isoform β promotes angiogenesis by TRiC-mediated upregulation of VEGF-A in lymphoma, Oncogene 41(28) (2022) 3655-3663.
[5] S. Inoue, K. Ikeda, K. Inoue, S. Kamada, S. Kawakami, Combination drug for treating kidney cancer and therapeutic effect enhancer for tyrosine kinase inhibitor, Saitama Medical University, Japan . 2022, p. 88pp.
[6] F. Liu, X. Xu, Compositions and methods for delivery of anticancer agents with improved therapeutic index, NanoTech Pharma Inc., USA . 2022, p. 111pp.
[7] Z. Wang, J. Chen, Q. Li, N. Li, J. Yu, F. Liu, The effects of axitinib plus tislelizumab in the treatment of advanced renal cell carcinoma, J. Oncol. (2022) 2700166.
[8] K. Su, Y. Peng, H. Yu, Development of a prognostic model based on pyroptosis-related genes in pancreatic adenocarcinoma, Dis. Markers (2022) 9141117.
[9] L. Zhai, M. Zhang, L. Lu, L. Li, Co-crystal of axitinib and saccharin [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 15pp.
[10] M. Zhang, L. Zhai, L. Li, C. Zhang, Axitinib saccharin co-crystal hydrate [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 14pp.
[11] L. Zhai, M. Zhang, X. Xia, L. Lu, Aoxitinib salt crystalline form and preparation method thereof [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 15pp.
[12] L. Zhai, M. Zhang, C. Zhang, Z. Zhu, Crystalline form of axitinib maleate and preparation thereof [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 17pp.
[13] M. Zhang, L. Zhai, X. Xu, J. Yu, Axitinib-vanillic acid co-crystal salt and preparation thereof [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 17pp.
[14] L. Zhai, M. Zhang, J. Liu, J. Wang, A crystalline form of axitinib citrate [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 14pp.
[15] L. Zhai, M. Zhang, C. Zhang, J. Wang, Co-crystal of axitinib and glutaric acid [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 15pp.
[16] M. Zhang, L. Zhai, J. Wang, J. Liu, Novel salt of axitinib malate [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 15pp.
[17] M. Zhang, L. Zhai, Z. Zhu, J. Wang, Z. Liu, Axitinib co-crystal salt [Machine Translation], Lunan Pharmaceutical Group Corporation, Peop. Rep. China . 2022, p. 16pp.
[18] J. Du, Y. Zhao, Q. Liu, L. Zhang, X. Wu, Q. Meng, Compound acting on abl 1 tyrosine kinase and application thereof [Machine Translation], Dalian University of Technology, Peop. Rep. China . 2022, p. 20pp.
[19] W. Li, Y. Yao, L. Yang, S. Zhang, Reagent and method for treating skin diseases or conditions associated with anti-tumor agent, OnQuality Pharmaceuticals China Ltd., Peop. Rep. China . 2022, p. 89pp.
[20] G.D. Stewart, S.J. Welsh, S. Ursprung, F.A. Gallagher, J.O. Jones, J. Shields, C.G. Smith, T.J. Mitchell, A.Y. Warren, A. Bex, E. Boleti, J. Carruthers, T. Eisen, K. Fife, A. Hamid, A. Laird, S. Leung, J. Malik, I.A. Mendichovszky, F. Mumtaz, G. Oades, A.N. Priest, A.C.P. Riddick, B. Venugopal, M. Welsh, K. Riddle, L.E.M. Hopcroft, T.G. Naxiva, R.J. Jones, A Phase II study of neoadjuvant axitinib for reducing the extent of venous tumour thrombus in clear cell renal cell cancer with venous invasion (NAXIVA), Br. J. Cancer (2022) Ahead of Print.
[21] T.Z. Zhuang, K. Case, T.A. Olsen, J.T. Brown, B.C. Carthon, O. Kucuk, J. Goldman, W. Harris, M.A. Bilen, B. Nazha, Metastatic Clear-Cell Renal Cell Carcinoma in the Era of Immune Checkpoint Inhibitors: Therapies and Ongoing Trials, Cancers 14(12) (2022) 2867.
[22] S. Prasanthi, G. Himabindu, Assay of bio analytical method for the estimation of avelumab and axitinib using UPLC and its application to pharmacokinetic studies, J. Pharm. Res. Int. 34(27A) (2022) JPRI.84631.
[23] C. Chen, Z. Jin, X. Xu, C. Zhang, B. Wu, T. Xu, Safety comparison of axitinib and everolimus in the treatment of renal cancer:analysis of adverse events based on pharmacovigilance database, Zhongliu Yaoxue 11(4) (2021) 7-15.
[24] X. Meng, L. Liu, Efficacy and safety of axitinib single agent or combined with pembrolizumab in the treatment of renal cell carcinoma with liver metastases after failure in sunitinib treatment, Zhongliu Yaoxue 11(6) (2021) 80-87.
You may like
Related articles And Qustion
See also
Lastest Price from Axitinib manufacturers

US $0.00-0.00/KG2025-09-12
- CAS:
- 319460-85-0
- Min. Order:
- 1KG
- Purity:
- USP
- Supply Ability:
- 100KG

US $5.00-0.50/KG2025-06-05
- CAS:
- 319460-85-0
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS