The important applications of salicylaldehyde
Introduction
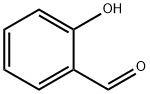
Salicylaldehyde is an organic substance with the chemical formula H7H6O2. It is a colorless to yellow oily liquid with a burning smell and almond smell[1]. Salicylaldehyde could be slightly soluble in water and other organic solvents. Salicylaldehyde is a kind of perfume and is widely used organic synthesis intermediate. Salicylaldehyde could be obtained by the reaction of phenol and chloroform in sodium hydroxide solution. Salicylaldehyde can volatilize with steam. When reacts with sulfuric acid, Salicylaldehyde can form colored chelate ring coordination compounds with metal ions and turn orange red. Salicyl alcohol is formed during reduction. Salicylaldehyde is often used to synthesize salinazid, benzbromarone and acetoamine benzophenone.

Picture 1 Salicylaldehyde liquid
Properties
Salicylaldehyde is a key precursor to a variety chelating agent, some of which are commercially important, salicylaldehyde is a common highly functionalized arene that has often been exploited as a precursor to still other chemical. Salicylaldehyde is converted to chelating ligands by condensation with amines. With ethylenediamine, it condenses to give the ligand salen. Hydroxylamine gives salicylaldoxime. Oxidation with hydrogen peroxide gives catechol (1,2-dihydroxybenzene) (Dakin reaction). Condensation with diethyl malonate gives a derivative of the heterocycle coumarin via an aldol condensation.
Salicyaldehyde and their derivatives can be good chelating ligands when they condensed with amines in 1:1 and 2:1 ratio to form bi, tri and tetra dentate Schiff base ligands suitable to form complexes with Metal ions. These Metal-Schiff base complexes have been shown to exhibita broad range of biological activities, including antifungal, antibacterial, antimalarial, anti-proliferative, anti inflammatory, antiviral, and antipyretic properties.
Salicyaldehyde and their derivatives can be good chelating ligands when they condensed with amines in 1:1 and 2:1 ratio to form bi, tri and tetra dentate Schiff base ligands suitable to form complexes with Metal ions. These Metal-Schiff base complexes have been shown to exhibit a broad range of biological activities, including antifungal, antibacterial, antimalarial, anti-proliferative, anti-inflammatory, antiviral, and antipyretic properties.
Application
Schiff bases are the most widely used organic com-pounds7 for industrial purposes and also exhibit a broad range of biological activities. Schiff base compounds and their metal complexes are very important as catalysts in various biological systems, polymers, dyes and medicinal and pharmaceutical fields4,8 they comprise miscellaneous therapeutically potent applications in the field of medicinal chemistry.9 Their use in birth control, food packages and as an O2 detector is also outlined8 Schiff’s bases chelates also used in quantitative analysis as an analytical chemical reagents and/or separation reagents have been also listed and discussed6 and synthetic applications in the field of the organic and inorganic chemistry. They have been shown to exhibit a broad range of bio-logical activities, including antifungal, antibacterial, antimalarial, antiproliferative, anti-inflammatory, antiviral, and antipyretic properties. Schiff’s base and their copper complexes possess remarkable properties as catalystsin various biological systems, polymers, dyes, antimicrobial activities, antifungal activities, antiviral activities insecticides, antitumor and cytotoxic activities, plant growth regulator, enzymatic activity and pharmaceutical fields.AvarietyofSchiff ’sbase and its complexes have been studied extensively. Severalmodelsystems, including those with bidentate, tridentate, tetradentate, multidentate Schiffbase ligands, and their coordination chemistry of copper attracts much attention because of its biological relevance and its own interesting coordination chemistry such as geometry, flexible redox property, and oxidation state.4 Gou and coworker used Salicylaldehyde base-Schiff as novel, easily available colorimetric and fluorescent double sensor. The sensor exhibits highly selective and sensitive recognition toward Cu+2 in aqueous solution via a naked eye color change from colorless to yellow and toward Al+3 via a significant fluorescent enhancement in ethanol over a wide range of tested metal ions. This represents the first reported Salicylaldehyde Schiff-based sensor capable of detecting Cu+2 and Al+3 using two different modes.
Schiff’s base and their copper complexes possess remark-able properties as catalystsin various biological systems, polymers, dyes, antimicrobial activities, antifungal activities, antiviral activities insecticides, antitumor and cytotoxic activities, plant growth regulator, enzymatic activity and pharmaceutical fields. Avarietyof Schiff ’s base and its complexes have been studied extensively. Severalmo delsystems, including those with bidentate, tridentate, tetradentate, multidentate Schiff base ligands, and their coordination chemistry of copper attracts much attention because of its biological relevance and its own interesting coordination chemistry such as geometry, flexible redox property, and oxidation state.4Gou and coworker used Salicylaldehyde base-Schiff as novel, easily available colorimetric and fluorescent double sensor. The sensor exhibits highly selective and sensitive recognition toward Cu+2 in aqueous solution via a naked eye color change from colorless to yellow and toward Al+3 via a significant fluorescent enhancement in ethanol over a wide range of tested metal ions. This represents the first reported Salicylaldehyde Schiff-based sensor capable of detecting Cu+2 and Al+3 using two different modes.
Reference
1 Shi, Lei, et al. "Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde." European journal of medicinal chemistry 42.4 (2007): 558-564.
2 Maher K A, Mohammed S R. Metal complexes of Schiff base derived from salicylaldehyde-A review[J]. International Journal of Current Research and Review, 2015, 7(2): 6.
See also
Lastest Price from Salicylaldehyde manufacturers

US $10.00/KG2025-04-21
- CAS:
- 90-02-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $0.00/KG2025-04-21
- CAS:
- 90-02-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt