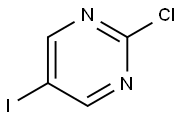
Intermediate role of 2-Chloro-5-iodopyrimidine in biological applications
2-Chloro-5-iodopyrimidine is 2-Chloro-5-iodopyridine is a halo-substituted pyridine, and it is used in biology application as synthesis intermediate in the multi-step synthesis [1].
The following examples can make understand easily.
It can synthesis 2-chloro-5-(2-pyridyl)pyrimidine, an intermediate in the synthesis of a selective phosphodiesterase type 5 (PDE-V) inhibitor by cross-coupling approach (Scheme 1). In the process, coupling of 2-pyridylzinc chloride and 2-chloro-5-iodopyrimidine in the presence of a catalytic amount of Pd(PPh3)4 was able to be achieved in 60–70% yield with a product purity for 2-chloro-5-(2-pyridyl)pyrimidine of greater than 95% [2].
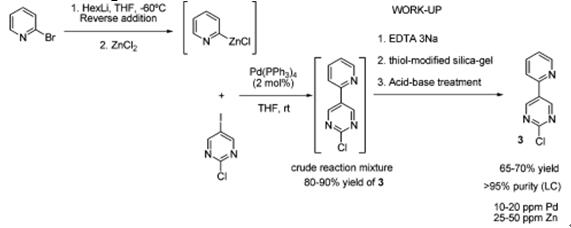
Scheme 1: The synthesis of 2-chloro-5-(2-pyridyl)pyrimidine
Laxmikant Atmaram Gharat and his colleagues synthesized the inhibitor of prostaglandin E synthetase (PGES-1) with 2-chloro-5-iodopyrimidine as intermediate [3]. 4-(5-iodopyrimidin-2-yl)morpholine is the intermediate of PGES inhibitors, which was synthesized with 3 steps as involving 2-chloro-5-iodopyrimidine inside. The first step is the preparation of 5-iodopyrimidine-2-amine. The second step is the preparation of 2-chloro-5-iodopyrimidine. In this step, a solution of 5-iodopyrimidine-2-amine in acetonitrile was added CuCI2 and tributyl nitrite. The reaction mass was heated at 70 ° C for 5-6 hours, then diluted with ether. After filtered, the obtained solid product was purified by Ethyl Acetate (EtOAc). The third synthesis step is the preparation of PGES inhibitor: 4-(5-iodopyrimidin-2-yl)morpholine. 2-chloro-5-iodopyrimidine was dissolved in morpholine and refluxed for 2-3 h. The reaction mass was quenched in water and the resulting solid was filtered, finally, the desired product was obtained after being dried.
In 1986, Japanese scientists synthesized N-benzoyl urea compounds, antitumorous compositions containing them as active ingredients, which was used to treat a cancer by administering these compounds. Therein, 2-chloro-5-iodopyrimidine was used to synthesis the N-benzoyl urea compound: N-(2-nitrobenzoyl)-N'-[4-fluoro-3-(5-iodo-2-pyrimidinyloxy)phenyl]urea. At the first step 2-chloro-5-iodopyrimidine, 2-fluorophenol, potassium carbonate and dimethylsulfoxide were put in the reactor and reacted at 100°C for 1 hour. After the completion of the reaction, the product was poured into water, and extracted with ethyl acetate. The extract was dried over anhydrous sodium sulfate, and the solvent was distilled off to obtain 2-(2-fluorophenoxy)-5-iodopyrimidine. Obtained 2-(2-fluorophenoxy)-5-iodopyrimidine was dissolved in sulfuric acid, and an acid mixture of nitric acid and sulfuric acid was slowly dropwise added at room temperature for the reaction. After purification, 2-(2-fluoro-5-nitrophenoxy)-5-iodopyrimidine was obtained. Then 2-(2-fluoro-5-nitrophenoxy)-5-iodopyrimidine was added to glacial acetic acid, and heated to 90°C, and then reduced iron was gradually added thereto. The mixture was refluxed for 5 minutes, and then returned to room temperature. A solvent mixture of acetone and water was added thereto, and 3-(5-iodo-2-pyrimidinyloxy)-4-fluoroaniline was got after purification and used in the final step. A solution of 3-(5-iodo-2-pyrimidinyloxy)-4-fluoroaniline in dioxane was added to 2-nitrobenzoylisocyanate, then the mixture was reacted at room temperature for 15 hours and get the final N-benzoyl urea compound after a series purification.
Reference
[1] https://www.sigmaaldrich.com/catalog/product/aldrich/498181?lang=en®ion=US
[2] C Pérez-Balado, A Willemsens, Development of a concise scaleable synthesis of 2-chloro-5-(pyridin-2-yl) pyrimidine via a Negishi cross-coupling, Organic Process Research and Development, 2007, vol. 11, # 2 p. 237 – 240
[3] Laxmikant Atmaram Gharat etc., Triazolone compound as an inhibitor of mPGES-1, TWI568722B
[4] Haga Takahiro, N-benzoyl urea compounds, antitumorous compositions containing them, and process for their preparation, EP0226104B1
See also
Lastest Price from 2-Chloro-5-iodopyrimidine manufacturers

US $1.60-6.60/kg2025-06-19
- CAS:
- 32779-38-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/KG2025-06-04
- CAS:
- 32779-38-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS