How Is Silver Bromide Used in Photography?
The Structure of Silver Bromide
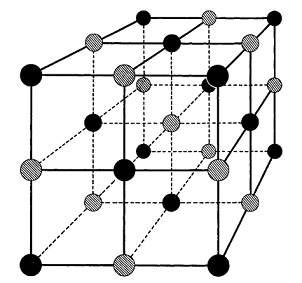
A crystal of silver bromide consists of an orderly array of silver and bromine in what is known as a cubical structure. Fig. 98 illustrates this structure in which the silver and bromine are seen to be at the corners of (imaginary) cubes. It must be noted that these are silver and bromine ions and not atoms. When the atoms combine to form silver bromide the electron from the outer shell of the silver atom moves over to the bromine and so gives it an overall negative charge (—e). The loss of the electron from the silver makes it have an overall positive charge (+e). Even in such a tiny crystal as occurs in the emulsion, this regular pattern of ions is repeated many, many times to produce the already-mentioned overall crystal shape. This description is of a perfect crystal which would be, in fact, of no use photographically. Fortunately, during the manufacture of the silver bromide, and in the subsequent preparation of the emulsion, faults of various kinds arise in the crystals.
These faults may be either of structure or due to the presence of a chemical contaminant. There have been many different suggestions about the exact identity of the contaminant responsible for the photographic property of silver bromide, but it is now commonly accepted that it is due to the sulphur compound, allyl thiocarbamide. Whatever it may be, the presence of this substance results in there being, on the surface of the crystal, a small region (Fig. 99 A) to which the name sensitivity speck is given, This region has the property that it is able to trap extra electrons and it is here that the black speck of silver will ultimately form. Apart from this small region the remainder of the surface of the crystal is composed of negatively charged bromine ions (Fig. 99 B). This does not mean, however, that the crystal as a whole is electrically charged, any more than the fact that there are electrons on the outside of atoms means that atoms are charged. Any excess of negative charge appearing on the crystal surface is exactly balanced by the presence of extra silver ions lying within (not at the corners of) the crystal lattice. These silver ions are called interstitial ions.
How Is Silver Bromide Used in Photography?
Silver bromide is used in photography as a component of an emulsion that helps develop a photographic image. Silver bromide is sensitive to light, and when suspended in gelatin, silver bromide's grains create a photographic emulsion. When exposed to light, silver bromide decomposes and as a result, it preserves a photographic image.
In 1874, J. Johnston and W.B. Bolton invented negative emulsion using silver bromide for chemical development of photographs. Within 4 years, Charles Bennett improved the method and the speed of developing the photographic image increased. The discovery was that when aged at 89.6 degrees Fahrenheit, the emulsion made with gelatin and silver bromide becomes more sensitive to light.
To use silver bromide in photography, it needs to be made into a photographic emulsion. This is formed on cellulose acetate, with the help of a thin layer of gelatin. Gelatin is needed to increase the emulsion's light sensitivity.
After silver bromide creates a photographic image, the image needs to be developed. Grains of silver bromide, which have reacted to light, become metallic silver, whereas those unaffected by light do not change. These remaining grains are washed away in a fixing solution.
The gelatin and silver bromide method of photograph development was an important step for astronomical photography, because it allowed objects that emit faint light to be captured on photographic film. Scientists used the silver bromide method to produce the first good images of Jupiter and Saturn during 1879 and 1886
You may like
Related articles And Qustion
Lastest Price from SILVER BROMIDE manufacturers

US $19.00/KG2025-04-15
- CAS:
- 7785-23-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg