General synthesis of phenols
Phenol, any of a family of organic compounds characterized by a hydroxyl (―OH) group attached to a carbon atom that is part of an aromatic ring. Besides serving as the generic name for the entire family, the term phenol is also the specific name for its simplest member, monohydroxybenzene (C6H5OH), also known as benzenol, or carbolic acid.
Phenols are similar to alcohols but form stronger hydrogen bonds. Thus, they are more soluble in water than are alcohols and have higher boiling points. Phenols occur either as colourless liquids or white solids at room temperature and may be highly toxic and caustic.

Phenols are widely used in household products and as intermediates for industrial synthesis. For example, phenol itself is used (in low concentrations) as a disinfectant in household cleaners and in mouthwash. Phenol may have been the first surgical antiseptic. In 1865 the British surgeon Joseph Lister used phenol as an antiseptic to sterilize his operating field. With phenol used in this manner, the mortality rate from surgical amputations fell from 45 to 15 percent in Lister’s ward. Phenol is quite toxic, however, and concentrated solutions cause severe but painless burns of the skin and mucous membranes. Less-toxic phenols, such as n-hexylresorcinol, have supplanted phenol itself in cough drops and other antiseptic applications. Butylated hydroxytoluene (BHT) has a much lower toxicity and is a common antioxidant in foods.
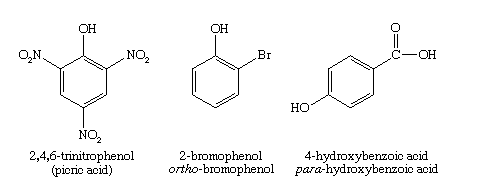
In industry, phenol is used as a starting material to make plastics, explosives such as picric acid, and drugs such as aspirin. The common phenol hydroquinone is the component of photographic developer that reduces exposed silver bromide crystals to black metallic silver. Other substituted phenols are used in the dye industry to make intensely coloured azo dyes. Mixtures of phenols (especially the cresols) are used as components in wood preservatives such as creosote.
Natural Sources Of Phenols
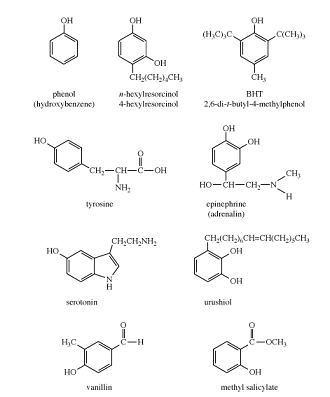
Phenols are common in nature; examples include tyrosine, one of the standard amino acids found in most proteins; epinephrine (adrenaline), a stimulant hormone produced by the adrenal medulla; serotonin, a neurotransmitter in the brain; and urushiol, an irritant secreted by poison ivy to prevent animals from eating its leaves. Many of the more complex phenols used as flavourings and aromas are obtained from essential oils of plants. For example, vanillin, the principal flavouring in vanilla, is isolated from vanilla beans, and methyl salicylate, which has a characteristic minty taste and odour, is isolated from wintergreen. Other phenols obtained from plants include thymol, isolated from thyme, and eugenol, isolated from cloves.

Phenol, the cresols (methylphenols), and other simple alkylated phenols can be obtained from the distillation of coal tar or crude petroleum.
Nomenclature Of Phenols
Many phenolic compounds were discovered and used long before chemists were able to determine their structures. Therefore, trivial names (i.e., vanillin, salicylic acid, pyrocatechol, resorcinol, cresol, hydroquinone, and eugenol) are often used for the most common phenolic compounds.
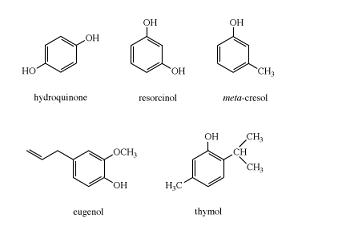
Systematic names are more useful, however, because a systematic name specifies the actual structure of the compound. If the hydroxyl group is the principal functional group of a phenol, the compound can be named as a substituted phenol, with carbon atom 1 bearing the hydroxyl group. For example, the systematic name for thymol is 5-methyl-2-isopropylphenol. Phenols with only one other substituent can be named using either the appropriate numbers or the ortho (1,2), meta (1,3), and para (1,4) system. Compounds with other principal functional groups can be named with the hydroxyl group as a hydroxy substituent. For example, the systematic name for vanillin is 4-hydroxy-3-methoxybenzaldehyde.
Physical Properties Of Phenols
Similar to alcohols, phenols have hydroxyl groups that can participate in intermolecular hydrogen bonding; in fact, phenols tend to form stronger hydrogen bonds than alcohols. (See chemical bonding: Intermolecular forces for more information about hydrogen bonding.) Hydrogen bonding results in higher melting points and much higher boiling points for phenols than for hydrocarbons with similar molecular weights. For example, phenol (molecular weight [MW] 94, boiling point [bp] 182 °C [359.6 °F]) has a boiling point more than 70 degrees higher than that of toluene (C6H5CH3; MW 92, bp 111 °C [231.8 °F]).
The ability of phenols to form strong hydrogen bonds also enhances their solubility in water. Phenol dissolves to give a 9.3 percent solution in water, compared with a 3.6 percent solution for cyclohexanol in water. The association between water and phenol is unusually strong; when crystalline phenol is left out in a humid environment, it picks up enough water from the air to form liquid droplets.
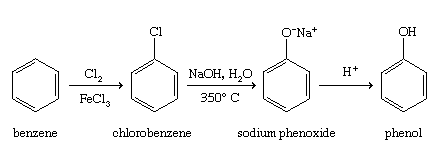
Hydrolysis of chlorobenzene (the Dow process)
Benzene is easily converted to chlorobenzene by a variety of methods, one of which is the Dow process. Chlorobenzene is hydrolyzed by a strong base at high temperatures to give a phenoxide salt, which is acidified to phenol.
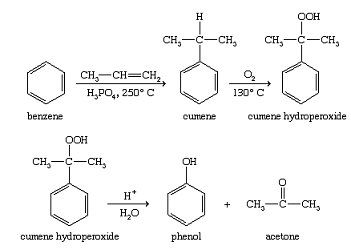
Oxidation of isopropylbenzene
Benzene is converted to isopropylbenzene (cumene) by treatment with propylene and an acidic catalyst. Oxidation yields a hydroperoxide (cumene hydroperoxide), which undergoes acid-catalyzed rearrangement to phenol and acetone. Although this process seems more complicated than the Dow process, it is advantageous because it produces two valuable industrial products: phenol and acetone.
General synthesis of phenols
To make more-complicated phenolic compounds, a more general synthesis is needed. The cumene hydroperoxide reaction is fairly specific to phenol itself. The Dow process is somewhat more general, but the stringent conditions required often lead to low yields, and they may destroy any other functional groups on the molecule. A milder, more general reaction is the diazotization of an arylamine (a derivative of aniline, C6H5NH2) to give a diazonium salt, which hydrolyzes to a phenol. Most functional groups can survive this technique, as long as they are stable in the presence of dilute acid.
Related articles And Qustion
See also
Lastest Price from Phenol manufacturers
US $100.00/kg2025-04-21
- CAS:
- 108-95-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $1746.00/T2025-03-26
- CAS:
- 108-95-2
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 1000T