Crystal Structure and Synthesis of Silver Bromide Complex
Synthesis
In the hitherto uninvestigated system silver(I) halide/dpm, authors have now discovered that specifically silver bromide forms a 1 : 1 complex (I) in which formally three monomer units unite with dissociation of a Br ion to give a novel Ag3Br2 structural moiety:
3 AgBr+3 dpm→(dpm)3Ag3Br2Br
The ligand CH2(PPh2)2 ("dprn") is characterized, like 1,8-naphthyridines and some phosphorus ylides, by the fact that the metal atoms in its dinuclear coordination compounds lie in very close proximity to one another, thus favoring a transition to metal-metal bonding. In dpm complexes of copper, aggregated groups are found to be present whose metal centers have a high tendency to fourfold coordination.
The product obtained on reaction of equimolar amounts of the reactants at 20°C in toluene crystallizes from CH2Cl2/pentane and shows the conductivity of a 1 : 1 electrolytein CH2Cl2.
Crystal Structure
The 'H-NMR spectrum contains, besides the phenyl multiplet, only one complex signal for the CH2 groups, indicating structural equivalence of all the dpm units. The structure and dimensions of the complex as deduced from an X-ray diffraction analysis are depicted in the figure below[1].
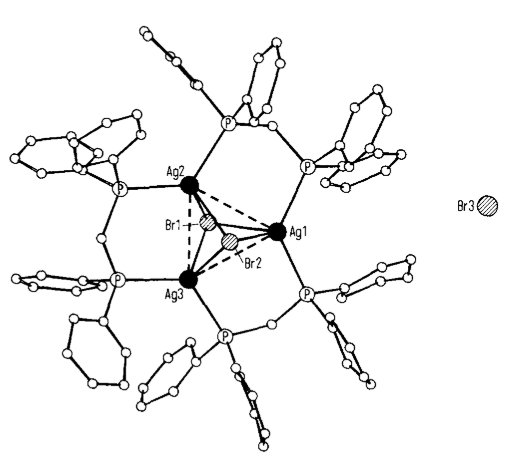
The silver atoms in (I) form an almost equilateral triangle; together with two approximately equidistant bromine atoms there arises a trigonal bipyramidal central unit. The sides of the Ag3 triangle are singly bridged by a dpm ligand whose phenyl groups are twisted to avoid steric hinderance. In such an arrangement the silver atoms have coordination number four, but the valence angles deviate somewhat from tetrahedral geometry. The two bromine atoms of the silver bromide cage are each bound to all three silver atoms (sharp trigonal pyramids with small AgBrAg angles), while the third bromine atom is present as an isolated Br ion. All in all, the structure contains six BrAg2P2C six-membered rings which are "condensed" via the Ag3Br2 skeleton in a chair or tub conformation. The metal-metal contacts of 319-336ppm may still be regarded as non-bonding.[1]
Reference
[1] Synthesis and Crystal Structure of a Methylenebis(diphenylphosphane) Complex of Silver Bromide Containing a Trigonal Bipyramidal Ag3Br2 Central Unit. Angew. Chem. Int. Ed. Engl. 17 125 (1978) No. 2.
You may like
Related articles And Qustion
See also
Lastest Price from SILVER BROMIDE manufacturers

US $19.00/KG2025-04-15
- CAS:
- 7785-23-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg