How is Piflufolastat F-18 synthesised?
Synthesis of Piflufolastat F-18
Piflufolastat F-18 is synthesised from Trimethylammonium Salt and Activated Ester through a series of chemical reactions. The specific synthesis steps are as follows:
Step 1: Synthesis of Trimethylammonium Salt 29.7 and Activated Ester 29.6
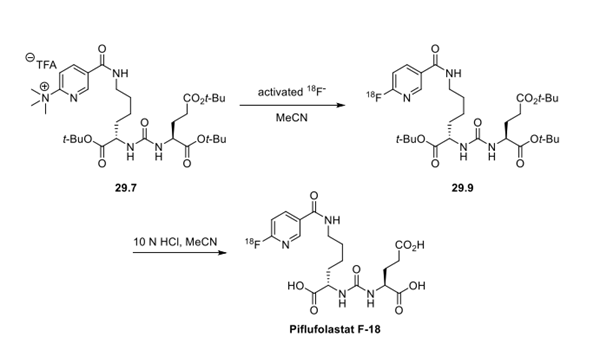
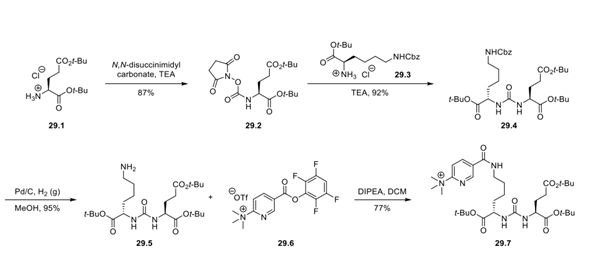
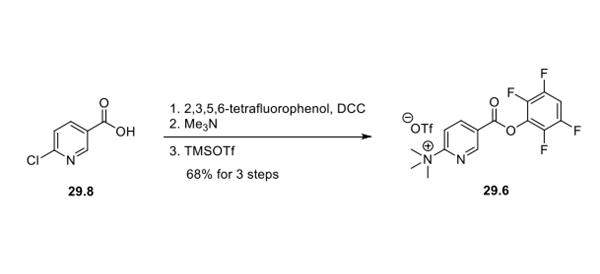
Because of the short half-life of the 18F isotope, the tracer must be generated on-site and used immediately in patients, necessitating a late-stage fluorination strategy. The fluorination precursor, trimethylammonium salt 29.7, was assembled in a convergent fashion. Di-t-butyl glutamate hydrochloride (29.1) was treated with N,N-disuccinimidyl carbonate to form carbamate 29.2, which was then coupled with amino ester hydrochloride 29.3 to afford urea 29.4. Cbz cleavage via hydrogenolysis delivered free amine 29.5. Coupling of amine 29.5 and activated ester 29.6, synthesized in a 3-step sequence from 6-chloronicotinic acid 29.8, forged the amide bond and generated trimethylammonium salt 29.7 as the fluorination precursor.
Step 2: Radiosynthesis of Piflufolastat F18
With fluorination precursor 29.7 in hand, the authors developed an automated radiosynthesis of 18F-labeled piflufolastat. Treatment of 29.7 with activated 18F− in acetonitrile at 60 °C for 10 min converted the trimethylammonium group to the 18-fluoride cleanly. Next, the tert-butyl esters were cleaved rapidly under acidic conditions. pH adjustment and HPLC purification afforded piflufolastat F18. The total synthesis time including HPLC purification was 55 min, and the overall isolated radiochemical yield of piflufolastat F18 was 23 ± 5% (n = 10, decay-corrected).