Asciminib Hydrochloride: Synthesis and Introduction
Synthesis of Asciminib Hydrochloride
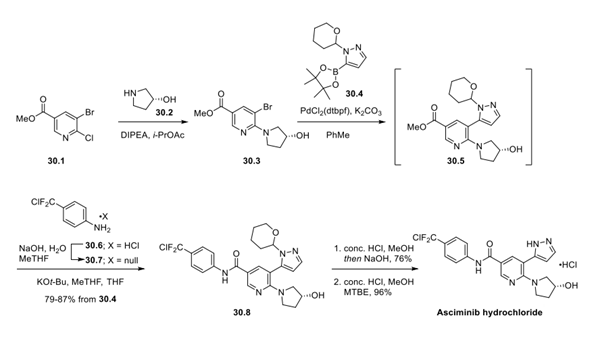
Asciminib Hydrochloride is synthesised using chloronicotinate as a raw material by chemical reaction. The specific synthesis steps are as follows:
30.3 can be prepared by SNAr reaction of chiral pyrrolidinol 30.2 with chloronicotinate 30.1, as previously disclosed. Suzuki cross-coupling of 30.3 with commercially sourceable boronic ester 30.4 was demonstrated on a 50 kg scale to provide pyrazole nicotinate 30.5, which was subsequently treated with the free base of aniline 30.6 in the presence of KOtBu to give 30.8 in 79−87% yield from boronic ester 30.4. Tetrahydropyranyl deprotection was achieved by exposure to 37% HCl solution in MeOH, and the free base was crystallized after pH adjustment with sodium hydroxide in 76% yield. Asciminib hydrochloride was then generated by the addition of HCl and crystallized from MeOH and MTBE in excellent yield.
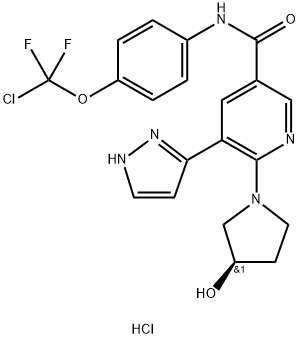
Introduction of Asciminib Hydrochloride
Discovered and developed by Novartis for the treatment of hematological malignancies including Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML), asciminib was the first allosteric BCR-ABL1 inhibitor to reach the clinic. Unlike conventional tyrosine kinase inhibitors (TKIs) that bind to the ATP-binding site, asciminib targets the myristoyl pocket of the BCR-ABL1 tyrosine kinase. It has demonstrated potent activity against several single catalytic site mutations, including T315I, that have caused resistance to conventional TKIs. In October 2021, asciminib was granted accelerated approval for the treatment of adults with Ph+ CML in chronic phase (CMLCP) that have previously been treated with at least 2 TKIs and full approval for the treatment of adults with Ph+ CML-CP with the T315I mutation by the USFDA.